Answered step by step
Verified Expert Solution
Question
1 Approved Answer
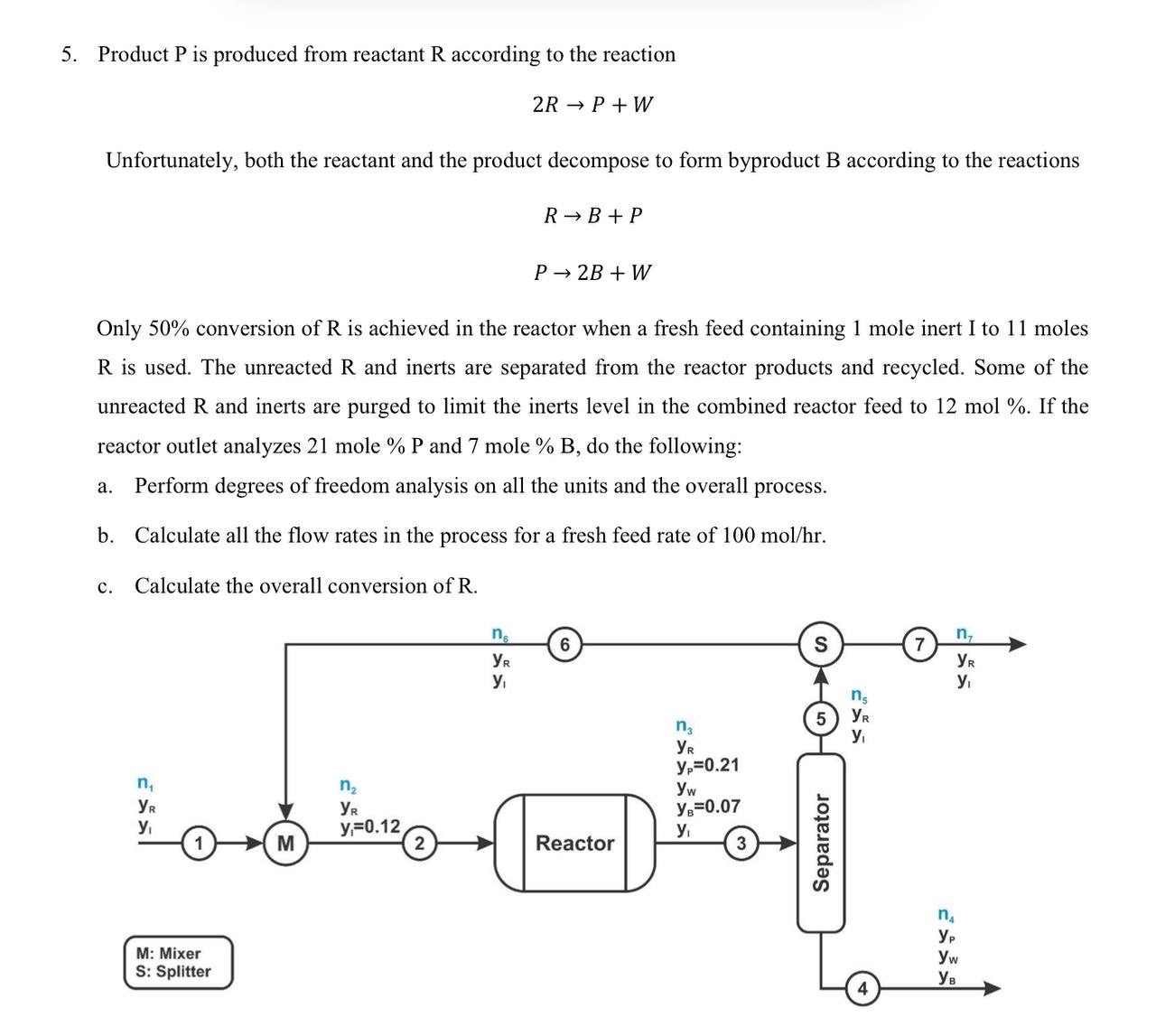
Product P is produced from reactant R according to the reaction 2 R P + W Unfortunately, both the reactant and the product decompose to
Product is produced from reactant according to the reaction
Unfortunately, both the reactant and the product decompose to form byproduct B according to the reactions
Only conversion of is achieved in the reactor when a fresh feed containing mole inert I to moles is used. The unreacted and inerts are separated from the reactor products and recycled. Some of the unreacted and inerts are purged to limit the inerts level in the combined reactor feed to mol If the reactor outlet analyzes mole and mole do the following:
a Perform degrees of freedom analysis on all the units and the overall process.
b Calculate all the flow rates in the process for a fresh feed rate of
c Calculate the overall conversion of
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


