Answered step by step
Verified Expert Solution
Question
1 Approved Answer
production of styrene from dehydrogenation of ethylbenzene, You are required to manufacture 3 0 0 0 0 kg h - 1 styrene, using catalytic dehydrogenation
production of styrene from dehydrogenation of ethylbenzene, You are required to manufacture kg h styrene, using catalytic dehydrogenation of EB I want material balance mol EB converted in reactor mol styrene yield mol of the EB that is converted forms benzene and ethane CHCHCH H CH CH mol of the EB that is converted forms methylbenzene and methane CHCHCH H CHCH CH mol of the EB that is converted forms tars through pyrolysis, which have very high but unknown RMM CHCHCH Tars All H CH and CH leave as gases wt benzene, wt toluene, wt EB and wt styrene entering the separator leaves with the gases. All water leaves separately assume complete immiscibility of organics During distillation, as the styrene product is heated, some of it polymerises assume wt of the styrene in each column does this These polymers and the tars are nonvolatile and finish up in the bottom product of any column in the train wt of styrene entering each column polymerises. Topping Column All remaining styrene in bottom product. All benzene entering leaves in the top product wt of the top product is EB wt of the bottom product is toluene Ethylbenzene Column All toluene entering leaves in the top product wt of top product is styrene wt of bottom product is EB 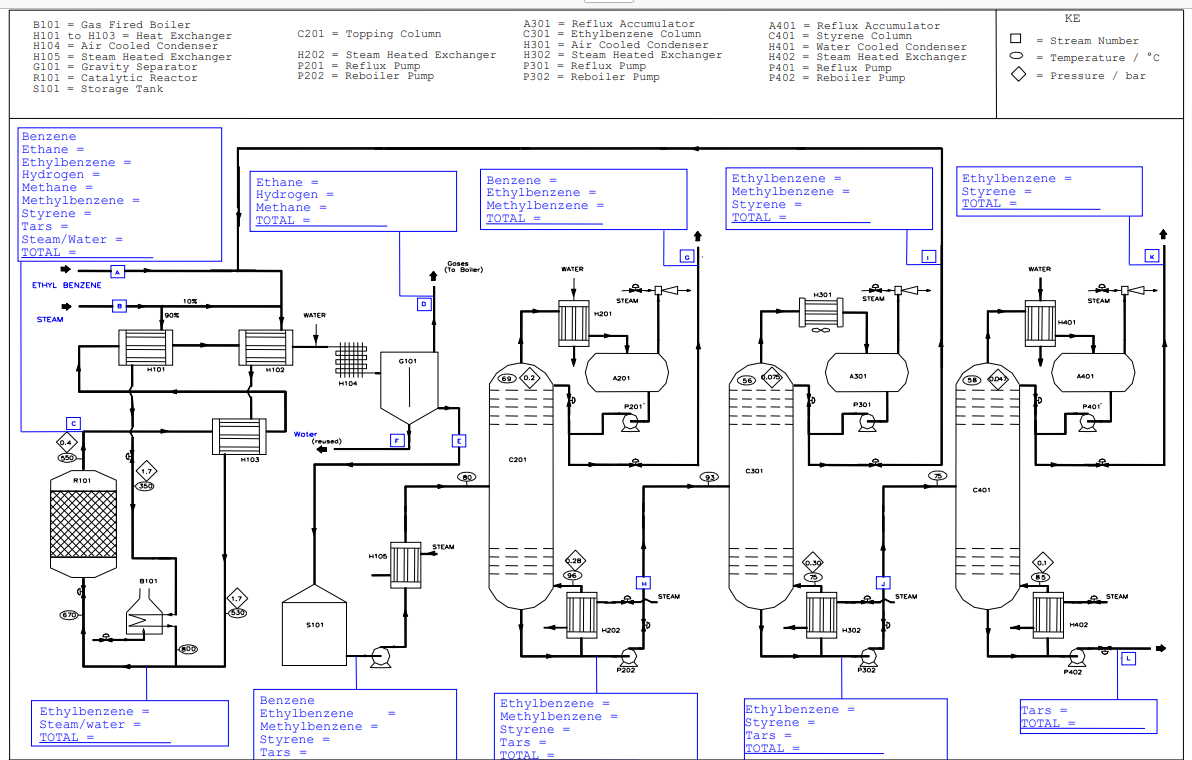
production of styrene from dehydrogenation of ethylbenzene, You are required to manufacture kg h styrene, using catalytic dehydrogenation of EB I want material balance mol EB converted in reactor mol styrene yield
mol of the EB that is converted forms benzene and ethane
CHCHCH H CH CH
mol of the EB that is converted forms methylbenzene and methane
CHCHCH H CHCH CH
mol of the EB that is converted forms tars through pyrolysis, which have very high but unknown RMM
CHCHCH Tars
All H CH and CH leave as gases
wt benzene, wt toluene, wt EB and wt styrene entering the separator leaves with the gases.
All water leaves separately assume complete immiscibility of organics
During distillation, as the styrene product is heated, some of it polymerises assume wt of the styrene in each column does this These polymers and the tars are nonvolatile and finish up in the bottom product of any column in the train wt of styrene entering each column polymerises.
Topping Column
All remaining styrene in bottom product.
All benzene entering leaves in the top product
wt of the top product is EB
wt of the bottom product is toluene
Ethylbenzene Column
All toluene entering leaves in the top product
wt of top product is styrene
wt of bottom product is EB
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


