Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Prove the following properties of Jacobians a) b) (u, v) a(x, v) (u, v) (x, y) = - (3) = (u, v) (a, b)
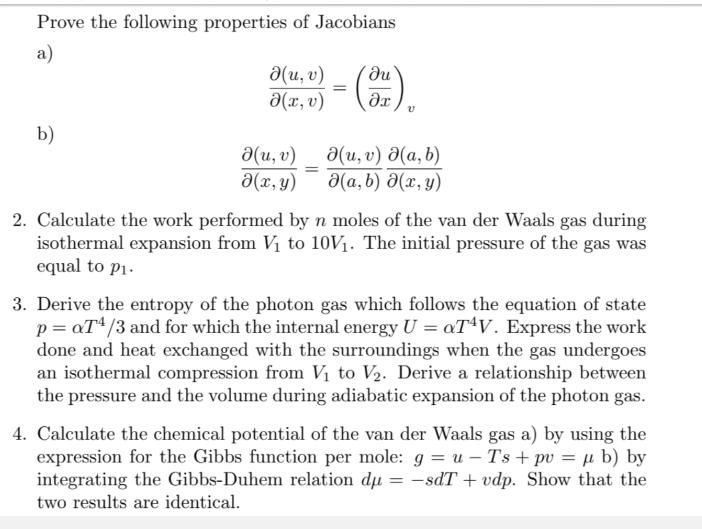
Prove the following properties of Jacobians a) b) (u, v) a(x, v) (u, v) (x, y) = - (3) = (u, v) (a, b) V (a, b) (x, y) 2. Calculate the work performed by n moles of the van der Waals gas during isothermal expansion from V to 10V. The initial pressure of the gas was equal to pi. 3. Derive the entropy of the photon gas which follows the equation of state p=aT4/3 and for which the internal energy U = aT4V. Express the work done and heat exchanged with the surroundings when the gas undergoes an isothermal compression from V to V. Derive a relationship between the pressure and the volume during adiabatic expansion of the photon gas. 4. Calculate the chemical potential of the van der Waals gas a) by using the expression for the Gibbs function per mole: gu - Ts + pv = b) by integrating the Gibbs-Duhem relation du = sdT + vdp. Show that the two results are identical.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


