Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Provide explanations for why it is or isn't true. If it's not true, provide the correct formula and describe why that's the correct formula (and/or
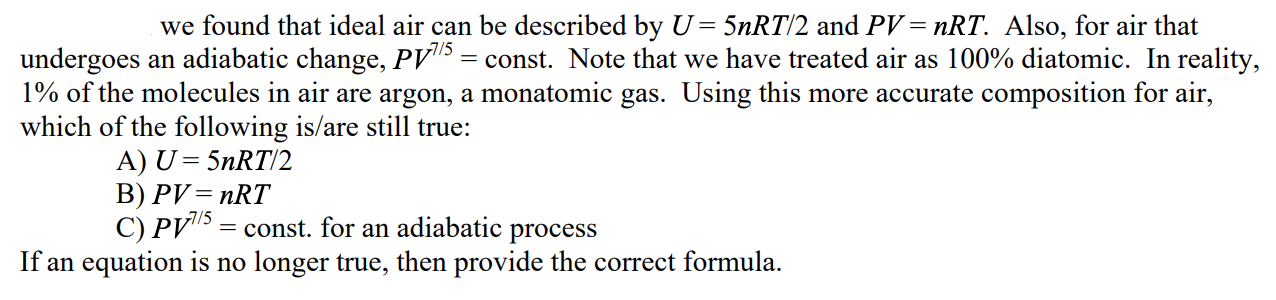
Provide explanations for why it is or isn't true. If it's not true, provide the correct formula and describe why that's the correct formula (and/or show derivations).
we found that ideal air can be described by U=5nRT/2 and PV=nRT. Also, for air that undergoes an adiabatic change, PV7/5= const. Note that we have treated air as 100% diatomic. In reality, 1% of the molecules in air are argon, a monatomic gas. Using this more accurate composition for air, which of the following is/are still true: A) U=5nRT/2 B) PV=nRT C) PV7/5= const. for an adiabatic process If an equation is no longer true, then provide the correct formulaStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


