Question
pulverized in a ball mill and the resulting powder was digested with various strong resulting solution was evaporated to dryness dissolved in dilute hydrochloric acid
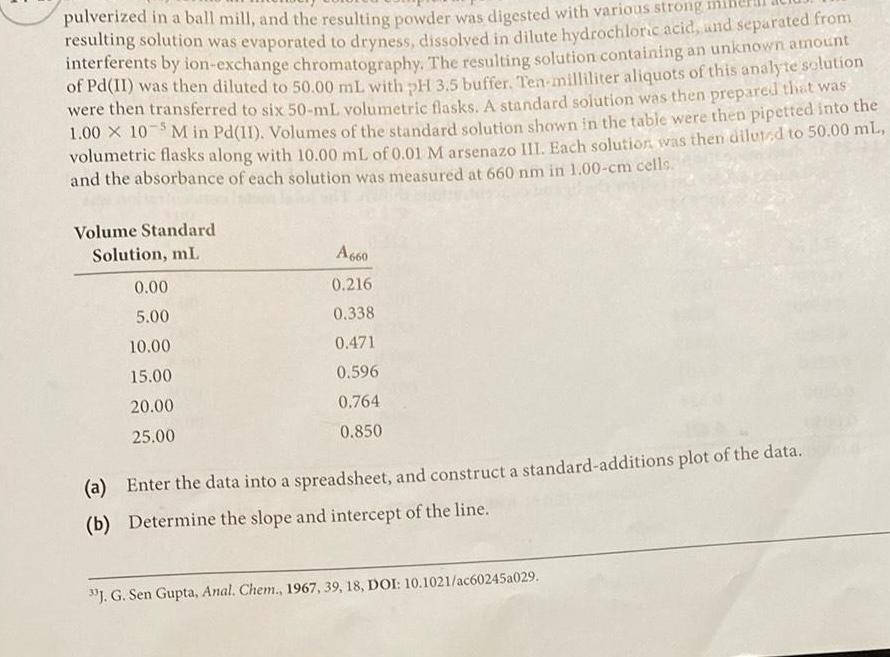
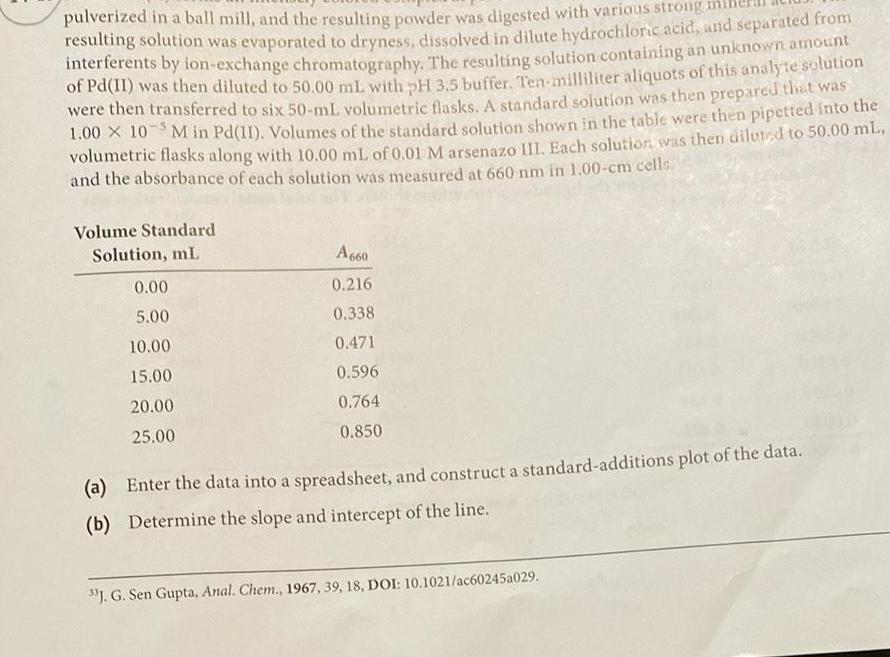
pulverized in a ball mill and the resulting powder was digested with various strong resulting solution was evaporated to dryness dissolved in dilute hydrochloric acid and separated from interferents by ion exchange chromatography The resulting solution containing an unknown amount of Pd II was then diluted to 50 00 mL with pH 3 5 buffer Ten milliliter aliquots of this analyte solution were then transferred to six 50 mL volumetric flasks A standard solution was then prepared that was 1 00 10 5M in Pd 11 Volumes of the standard solution shown in the table were then pipetted into the volumetric flasks along with 10 00 mL of 0 01 M arsenazo III Each solution was then diluted to 50 00 mL and the absorbance of each solution was measured at 660 nm in 1 00 cm cells Volume Standard Solution mL 0 00 5 00 10 00 15 00 20 00 25 00 A660 0 216 0 338 0 471 0 596 0 764 0 850 a Enter the data into a spreadsheet and construct a standard additions plot of the data b Determine the slope and intercept of the line J G Sen Gupta Anal Chem 1967 39 18 DOI 10 1021 ac60245a029
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


