Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Pure S is burned in dry air. The flue gas has the following composition: 8.17 mole% SOz. 0.90% molar SOs, 11.57% molar 02 and 79.36%
Pure S is burned in dry air. The flue gas has the following composition: 8.17 mole% SOz. 0.90% molar SOs, 11.57% molar 02 and 79.36% molar N2. Calculate the percentage in excess of oxygen that has been used with respect to that necessary for the complete combustion to SO3.
The base of calculus is 100 mol.
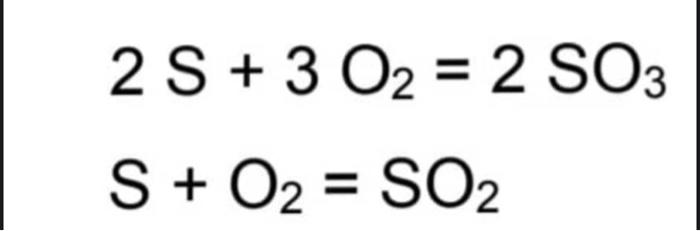
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


