Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Pure water is used to dilute 1 9 2 l b m of a sulfuric acid stream that has an initial mass fraction of H
Pure water is used to dilute of a sulfuric acid stream that has an initial mass fraction of equal to in order to
produce a diluted acid with an mass fraction of The temperature of the water is and that of the concentrated acid is
An enthalpycomposition diagram for the system is shown below.
Enthalpyconcentration chart for Redrawn from the data of W D Ross, Chem. Eng. Progr., :
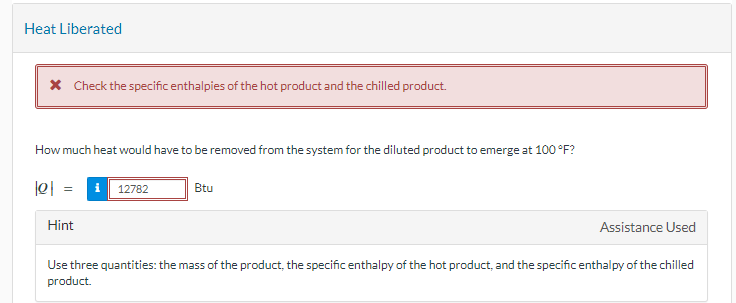
Heat Liberated
Check the specific enthalpies of the hot product and the chilled product.
How much heat would have to be removed from the system for the diluted product to emerge at
Btu
Hint
Assistance Used
Use three quantities: the mass of the product, the specific enthalpy of the hot product, and the specific enthalpy of the chilled
product.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


