Answered step by step
Verified Expert Solution
Question
1 Approved Answer
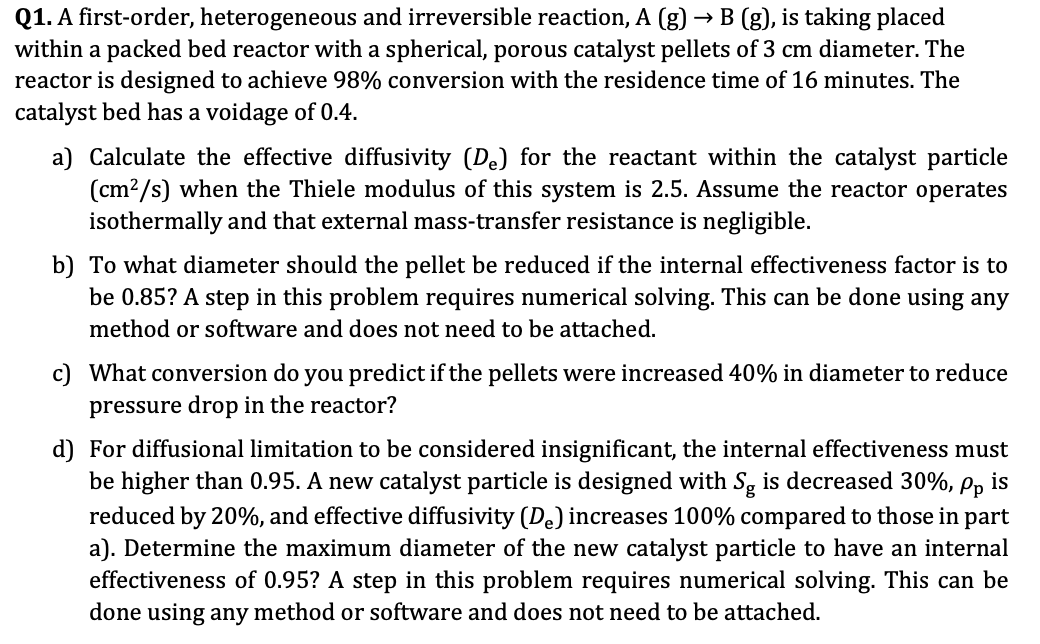
Q 1 . A first - order, heterogeneous and irreversible reaction, A ( g ) B ( g ) , is taking placed within a
Q A firstorder, heterogeneous and irreversible reaction, A is taking placed
within a packed bed reactor with a spherical, porous catalyst pellets of diameter. The
reactor is designed to achieve conversion with the residence time of minutes. The
catalyst bed has a voidage of
a Calculate the effective diffusivity for the reactant within the catalyst particle
when the Thiele modulus of this system is Assume the reactor operates
isothermally and that external masstransfer resistance is negligible.
b To what diameter should the pellet be reduced if the internal effectiveness factor is to
be A step in this problem requires numerical solving. This can be done using any
method or software and does not need to be attached.
c What conversion do you predict if the pellets were increased in diameter to reduce
pressure drop in the reactor?
d For diffusional limitation to be considered insignificant, the internal effectiveness must
be higher than A new catalyst particle is designed with is decreased is
reduced by and effective diffusivity increases compared to those in part
a Determine the maximum diameter of the new catalyst particle to have an internal
effectiveness of A step in this problem requires numerical solving. This can be
done using any method or software and does not need to be attached.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


