Answered step by step
Verified Expert Solution
Question
1 Approved Answer
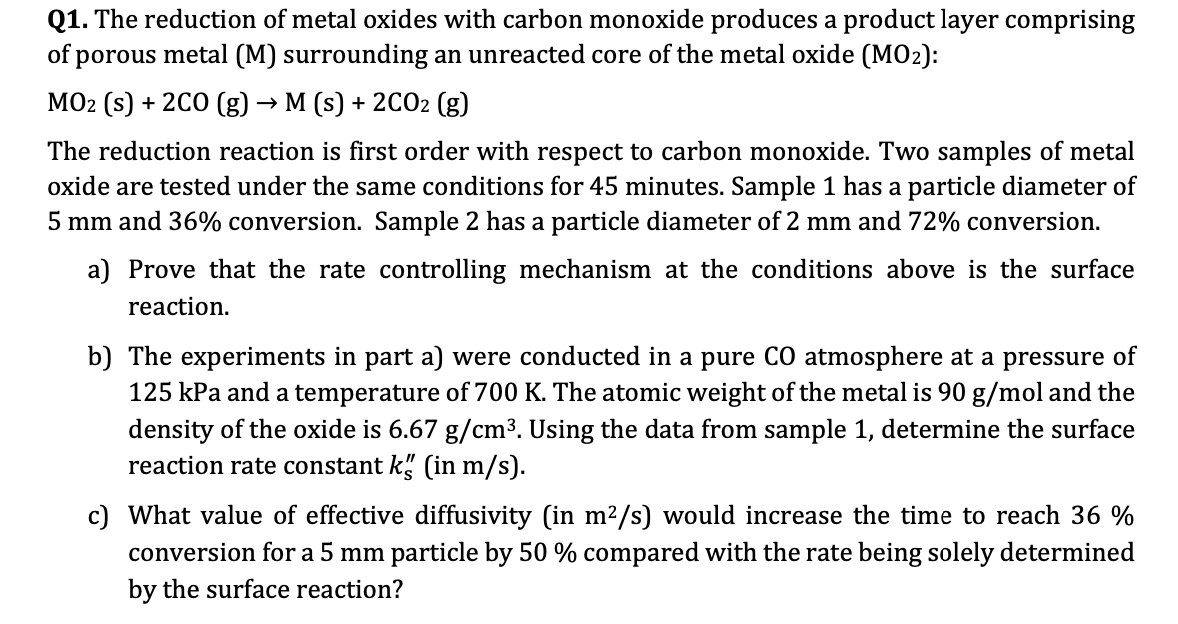
Q 1 . The reduction of metal oxides with carbon monoxide produces a product layer comprising of porous metal ( M ) surrounding an unreacted
Q The reduction of metal oxides with carbon monoxide produces a product layer comprising
of porous metal M surrounding an unreacted core of the metal oxide :
The reduction reaction is first order with respect to carbon monoxide. Two samples of metal
oxide are tested under the same conditions for minutes. Sample has a particle diameter of
and conversion. Sample has a particle diameter of and conversion.
a Prove that the rate controlling mechanism at the conditions above is the surface
reaction.
b The experiments in part a were conducted in a pure atmosphere at a pressure of
kPa and a temperature of The atomic weight of the metal is and the
density of the oxide is Using the data from sample determine the surface
reaction rate constant in
c What value of effective diffusivity in would increase the time to reach
conversion for a particle by compared with the rate being solely determined
by the surface reaction?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


