Answered step by step
Verified Expert Solution
Question
1 Approved Answer
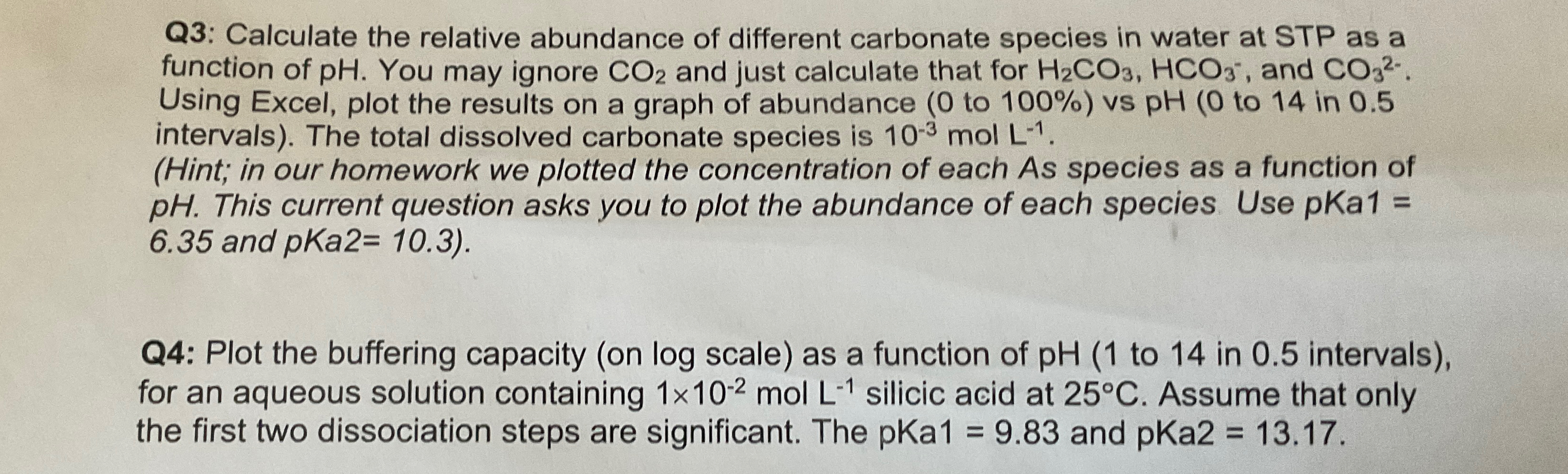
Q 3 : Calculate the relative abundance of different carbonate species in water at STP as a function of p H . You may ignore
Q: Calculate the relative abundance of different carbonate species in water at STP as a function of You may ignore and just calculate that for and Using Excel, plot the results on a graph of abundance to vs pH to in intervals The total dissolved carbonate species is
Hint; in our homework we plotted the concentration of each As species as a function of pH This current question asks you to plot the abundance of each species. Use pKa and pKa
Q: Plot the buffering capacity on log scale as a function of to in intervals for an aqueous solution containing silicic acid at Assume that only the first two dissociation steps are significant. The pKa and pKa
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


