Question: Q 6 . A sample consisting of 1 . 0 mol of perfect gas molecules with C V V = 2 0 . 8 J
Q A sample consisting of mol of perfect gas molecules with is initially at atm and It undergoes reversible adiabatic expansion until its pressure reaches atm. Calculate the final volume and temperature and the work done.
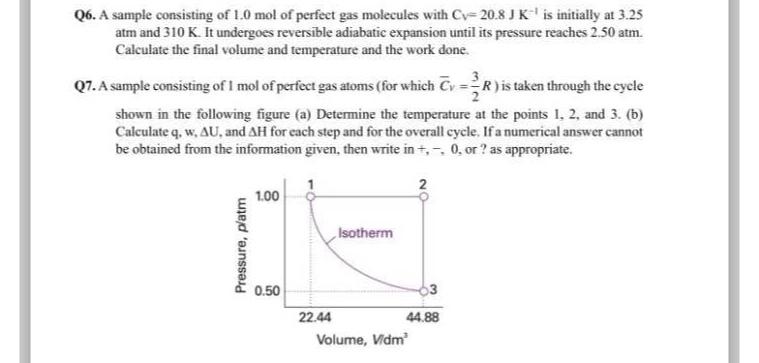
Q A sample consisting of mol of perfect gas atoms for which is taken through the cycle shown in the following figure a Determine the temperature at the points and b Calculate q w and for each step and for the overall cycle. If a numerical answer cannot be obtained from the information given, then write in or as appropriate.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


