Question: A hydrogen atom in an excited state can be ionized with less energy than when it is in its ground state. What is n
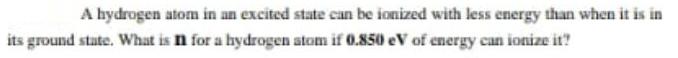
A hydrogen atom in an excited state can be ionized with less energy than when it is in its ground state. What is n for a hydrogen atom if 0.850 eV of energy can ionize it?
Step by Step Solution
★★★★★
3.45 Rating (164 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

Given The ionized energy or electron energy in the hydrogen a... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


