Question
Q1: [5 marks] a gas mixture containing (N2=10%, H-25%, NH3=60%, and Ar=5%) flows through a pipe, 25.4 mm in diameter, at 4.05 bar total
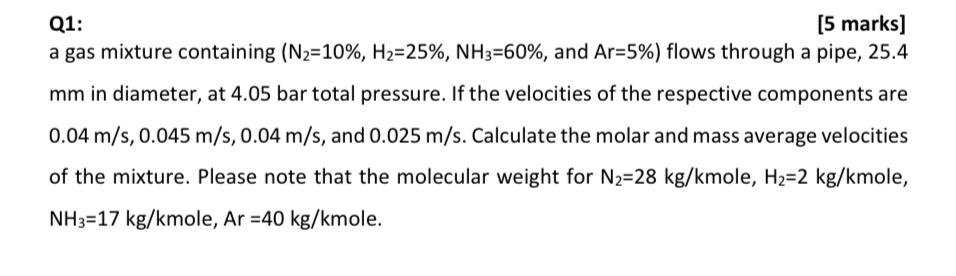
Q1: [5 marks] a gas mixture containing (N2=10%, H-25%, NH3=60%, and Ar=5%) flows through a pipe, 25.4 mm in diameter, at 4.05 bar total pressure. If the velocities of the respective components are 0.04 m/s, 0.045 m/s, 0.04 m/s, and 0.025 m/s. Calculate the molar and mass average velocities of the mixture. Please note that the molecular weight for N2-28 kg/kmole, H2=2 kg/kmole, NH3 17 kg/kmole, Ar =40 kg/kmole.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Manufacturing Processes for Engineering Materials
Authors: Serope Kalpakjian, Steven Schmid
5th edition
132272717, 978-0132272711
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



