Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q1. A proposed Olympic size swimming pool is maintained at a temperature of 28C throughout the year. It is estimated that, on average, the



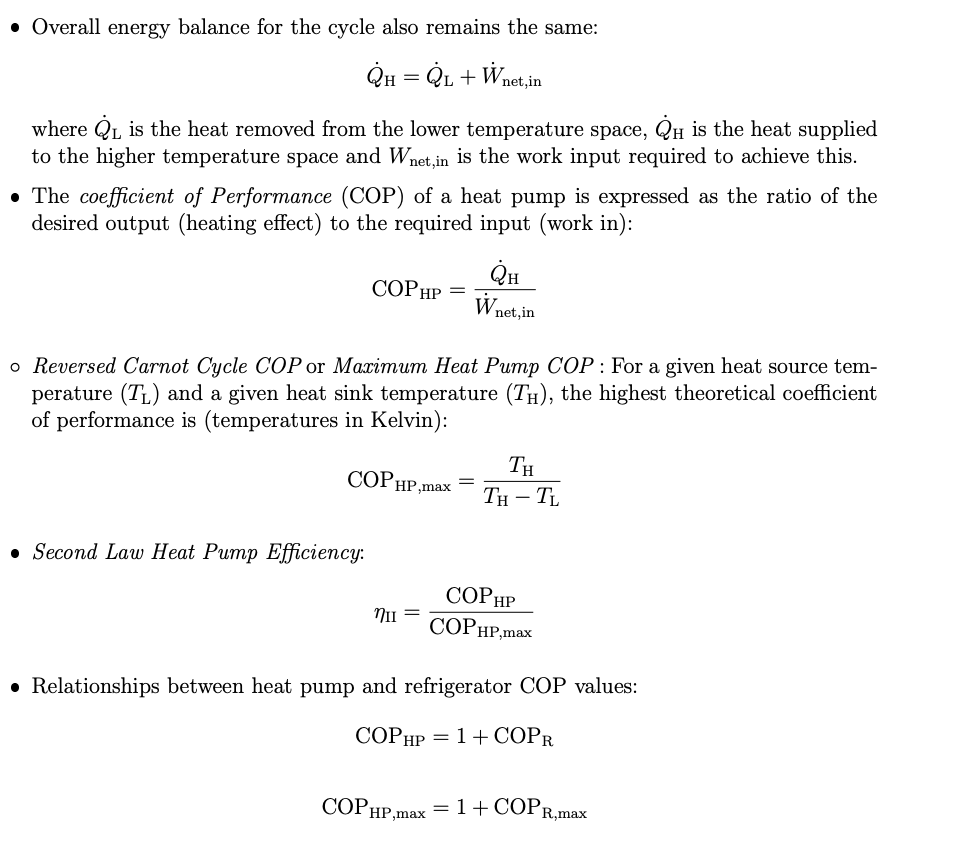
Q1. A proposed Olympic size swimming pool is maintained at a temperature of 28C throughout the year. It is estimated that, on average, the pool requires 120 kW of heating. To heat the pool, water is pumped from the pool through a solar heat exchanger, then a supplementary heating system, and back to the pool. An average of 45% of the heating will be supplied by an evacuated tube solar water heating system. For the remaining 55% of the heating required, there are three options: 1. A direct electric heater that is 97% efficient. The 3% loss is heat lost to the surroundings. 2. A gas-fired heater that is 85% efficient based on the HHV of the gas. The 15% loss is primarily through high-temperature exhaust gas, with some lost as heat to the surroundings. 3. A heat pump with an estimated COP of 2.9 and has the same 3% heat loss to the surroundings as the direct electric system. Electricity costs are $0.22/kWh and the life-cycle carbon intensity of the electricity is 520 kgCO2e/MWh. Gas costs $0.035/MJ and has a life-cycle carbon intensity of the methane of 70 kgCO2e/GJ. Determine: a) The annual MWh and GJ of electricity or gas used for each option. b) The annual electricity/gas heating cost of each option. c) The annual CO2e emissions associated with the electricity/gas use for each heating option. d) The annual CO2e emissions saved by using the solar heating instead of heating 100% with each option. e) Tabulate the results from (a) to (d). Q2. For the remaining questions, we assume a heat pump will be used to provide 55% of the heat needed. The conditions for the heat pump are: Operates on a vapor-compression cycle with refrigerant R134a. The outlet of the compressor is at 1.5 MPa and 68C. The outlet of the condenser is a saturated liquid at 55C. The inlet to the evaporator is at 11C. The outlet to the evaporator is at 420 kPa and 11C. The ambient temperature is 21C, and the pool water temperature is 28C. The inlet to the evaporator is at 11C. The outlet to the evaporator is at 420 kPa and 11C. The ambient temperature is 21C, and the pool water temperature is 28C. a) Illustrate the cycle with each stream numbered sequentially. Start with the evaporator outlet as stream 1. Show direction of flows and energy transfers. Highlight where heat is exchanged with the pool water and ambient air. Using the stream numbering, determine: b) The water flow rate recirculated through the heating system, knowing the water temperature rises by 2.5C. Assume water has a constant heat capacity of 4.18 kJ/kg.K. c) The work needed to pump the water through the heater system if the pressure drop is 160 kPa. d) The refrigerant flow rate required. Q3. For the heat pump in Q2, determine: a) The compressor work. b) The flow rate of air required for the evaporator, assuming air can only be cooled by 5C (use a constant heat capacity for air equal to its value at 305 K). c) The COP and second law efficiency of the heat pump. Overall energy balance for the cycle also remains the same: QH=QL + Wne where Q is the heat removed from the lower temperature space, QH is the heat supplied to the higher temperature space and Wnet,in is the work input required to achieve this. The coefficient of Performance (COP) of a heat pump is expressed as the ratio of the desired output (heating effect) to the required input (work in): COPHP = COP HP,max Second Law Heat Pump Efficiency: net, in o Reversed Carnot Cycle COP or Maximum Heat Pump COP: For a given heat source tem- perature (TL) and a given heat sink temperature (T), the highest theoretical coefficient of performance is (temperatures in Kelvin): NII QH W net, in TH TH - TL COPHP COPHP,max Relationships between heat pump and refrigerator COP values: COPHP = 1+ COPR COPHP,max = 1+ COPR,max
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


