Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q1) Carbon dioxide is produced by the gas-phase oxidation of naphthalene in a flow reactor. The feed consists of reactants enters at constant pressure of
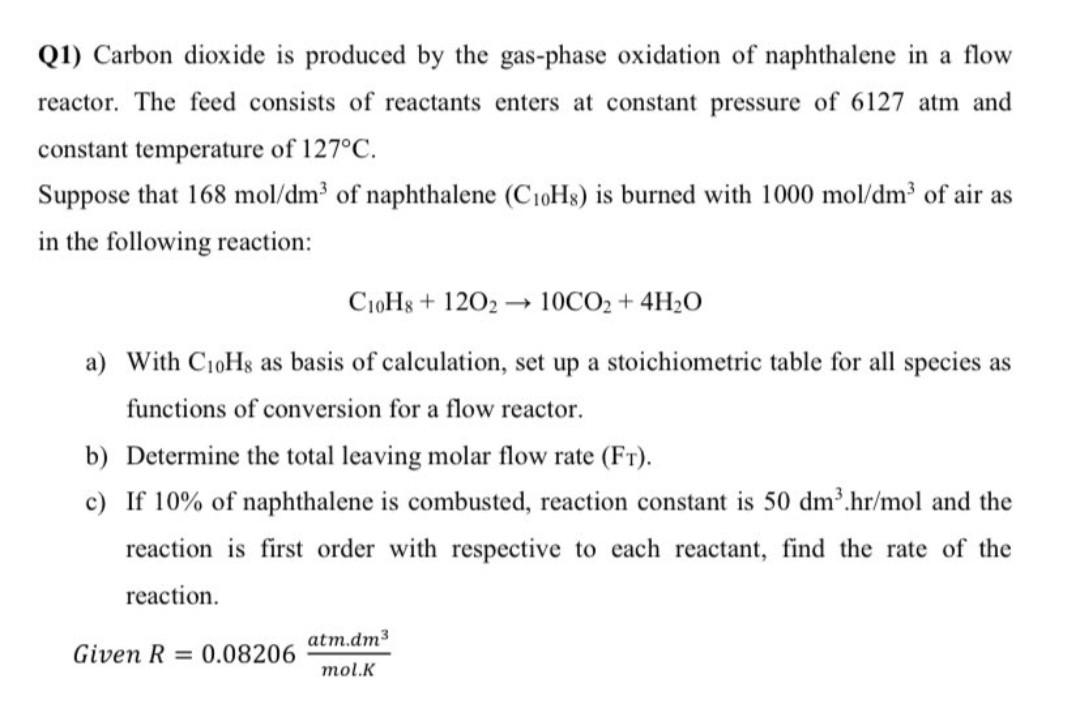
Q1) Carbon dioxide is produced by the gas-phase oxidation of naphthalene in a flow reactor. The feed consists of reactants enters at constant pressure of 6127 atm and constant temperature of 127C. Suppose that 168 mol/dm of naphthalene (C10H8) is burned with 1000 mol/dm3 of air as in the following reaction: C10H3 + 1202 10CO2 + 4H20 a) With C19Hg as basis of calculation, set up a stoichiometric table for all species as functions of conversion for a flow reactor. b) Determine the total leaving molar flow rate (Ft). c) If 10% of naphthalene is combusted, reaction constant is 50 dm'.hr/mol and the reaction is first order with respective to each reactant, find the rate of the reaction. atm.dm3 Given R = 0.08206 mol.K
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


