Answered step by step
Verified Expert Solution
Question
1 Approved Answer
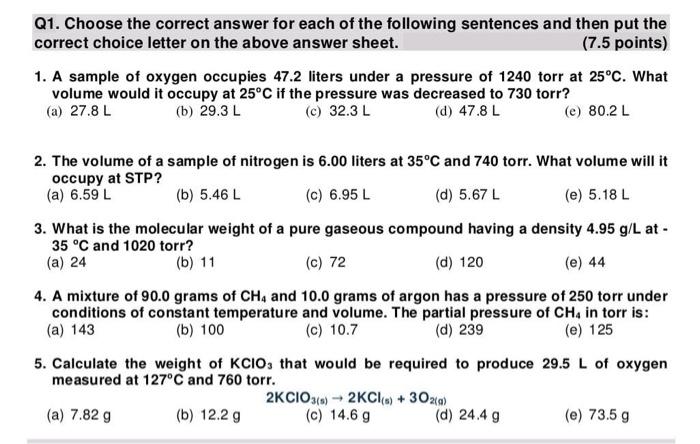
Q1. Choose the correct answer for each of the following sentences and then put the correct choice letter on the above answer sheet. (7.5 points)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


