Question
Q1: From the thermal death data for Clostridium cellulolyticum shown below, determine the frequency factor and activation energy of death for this cell line.
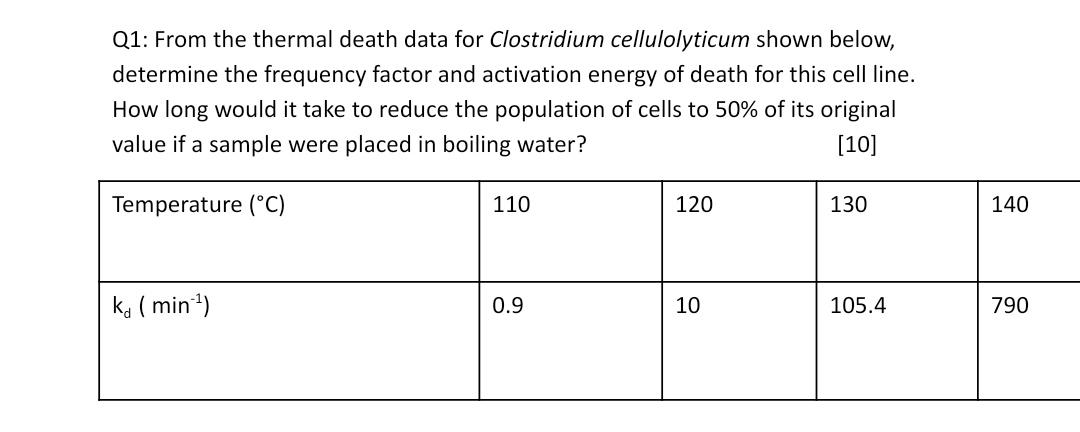
Q1: From the thermal death data for Clostridium cellulolyticum shown below, determine the frequency factor and activation energy of death for this cell line. How long would it take to reduce the population of cells to 50% of its original value if a sample were placed in boiling water? [10] Temperature (C) 110 120 130 140 ka ( min) 0.9 10 105.4 790
Step by Step Solution
3.40 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
Temperature Tin C Temperature T inK 1T k d min 1 lnk d slope intercept 110 38315 000260 09 010...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physics Principles with Applications
Authors: Douglas C. Giancoli
7th edition
978-0321869111, 321625927, 9780321733627, 321869117, 9780321625922, 321733622, 978-0321762429
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



