Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q1: In a particular installation, the purification system for the removal of sulfur compounds, designed to operate at a feed rate of up to 820
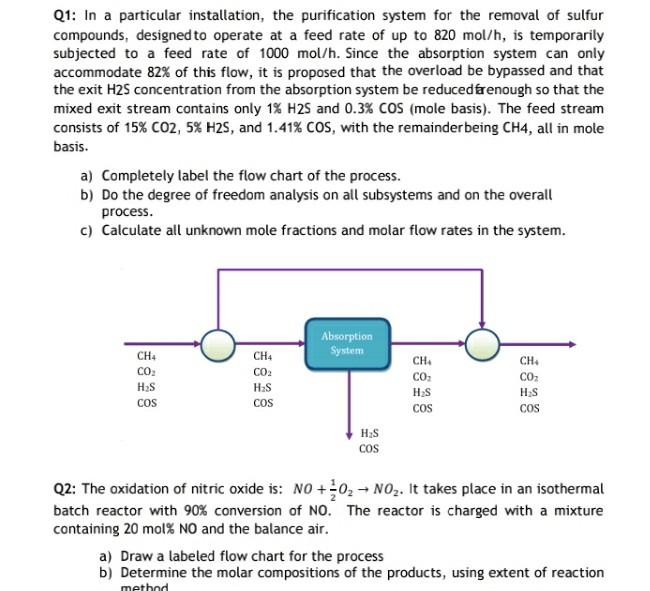
Q1: In a particular installation, the purification system for the removal of sulfur compounds, designed to operate at a feed rate of up to 820 mol/h, is temporarily subjected to a feed rate of 1000 mol/h. Since the absorption system can only accommodate 82% of this flow, it is proposed that the overload be bypassed and that the exit H2S concentration from the absorption system be reduced faenough so that the mixed exit stream contains only 1% H2S and 0.3% COS (mole basis). The feed stream consists of 15% CO2, 5% H2S, and 1.41% COS, with the remainder being CH4, all in mole basis. a) Completely label the flow chart of the process. b) Do the degree of freedom analysis on all subsystems and on the overall process. c) Calculate all unknown mole fractions and molar flow rates in the system. Absorption System CH CO HES COS CH4 CO HES COS CH CO HES COS CH CO2 HS COS H:S COS Q2: The oxidation of nitric oxide is: NO + 02 - NO2. It takes place in an isothermal batch reactor with 90% conversion of NO. The reactor is charged with a mixture containing 20 mol% NO and the balance air. a) Draw a labeled flow chart for the process b) Determine the molar compositions of the products, using extent of reaction method
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


