Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q1. out=33 psig D-100 P = 4 AP = 2 psi LAP = 4 [WI V-100 P = 10 psig P-100 HX-100 F-100 =
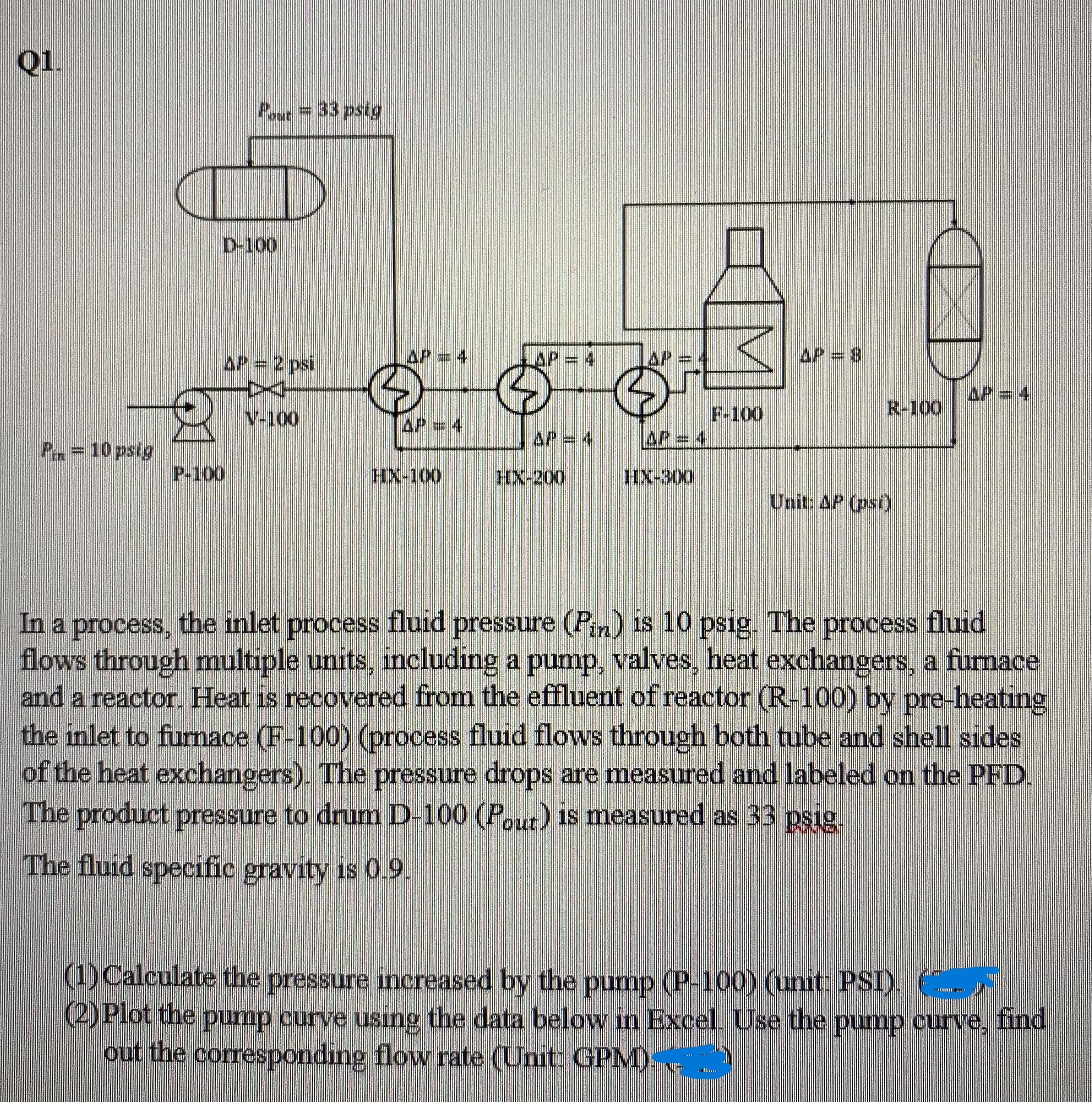

Q1. out=33 psig D-100 P = 4 AP = 2 psi LAP = 4 [WI V-100 P = 10 psig P-100 HX-100 F-100 = 4 AP = 4 HX-200 AP = 4 HX-300 = 8 APE 4 R-100 Unit: AP (psi) In a process, the inlet process fluid pressure (Pin) is 10 psig. The process fluid flows through multiple units, including a pump, valves, heat exchangers, a furnace and a reactor. Heat is recovered from the effluent of reactor (R-100) by pre-heating the inlet to furnace (F-100) (process fluid flows through both tube and shell sides of the heat exchangers). The pressure drops are measured and labeled on the PFD. The product pressure to drum D-100 (Pout) is measured as 33 psig. The fluid specific gravity is 0.9. (1) Calculate the pressure increased by the pump (P-100) (unit: PSI). (2) Plot the pump curve using the data below in Excel. Use the pump curve, find out the corresponding flow rate (Unit: GPM) Table 1. Pump (P-100) curve data: + Motor Speed-3500 rpm F(GPM) H(ft) 0 173 167 360 160 540 154 720 148 900 1080 136 1260 1440 019T 108 1800 195 0861 75 Q2. In P1, the normal motor speed for pump P-100 is 3500 rpm (curve data in Table 1). In a revamp project, a VFD is used to reduce the motor speed to 3150 rpm. (1) Plot both the original pump curve (n = 3500rpm) and the new pump curve (n = 3150 rpm) in the same plot using Excel (2) In the new process design (motor speed n is 3150 rpm), the process fluid flow rate is 486 GPM, assuming Pin and all the pressure drops (AP) are the same as in P1, what is Pout at the collection drum (D-100) under new process condition
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


