Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q1) Show that work done during the vaporization of 1 kg of saturated liquid water at 200 C can be calculated in two different
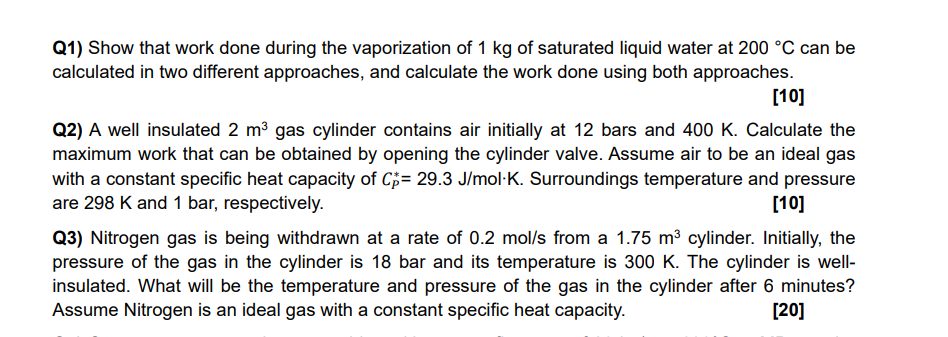
Q1) Show that work done during the vaporization of 1 kg of saturated liquid water at 200 C can be calculated in two different approaches, and calculate the work done using both approaches. [10] Q2) A well insulated 2 m gas cylinder contains air initially at 12 bars and 400 K. Calculate the maximum work that can be obtained by opening the cylinder valve. Assume air to be an ideal gas with a constant specific heat capacity of Cp = 29.3 J/mol K. Surroundings temperature and pressure are 298 K and 1 bar, respectively. [10] Q3) Nitrogen gas is being withdrawn at a rate of 0.2 mol/s from a 1.75 m cylinder. Initially, the pressure of the gas in the cylinder is 18 bar and its temperature is 300 K. The cylinder is well- insulated. What will be the temperature and pressure of the gas in the cylinder after 6 minutes? Assume Nitrogen is an ideal gas with a constant specific heat capacity. [20]
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


