Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q1. To heat up a solid alloy, at what temperature does melting start to occur? Q2. What is the composition of the first liquid phase
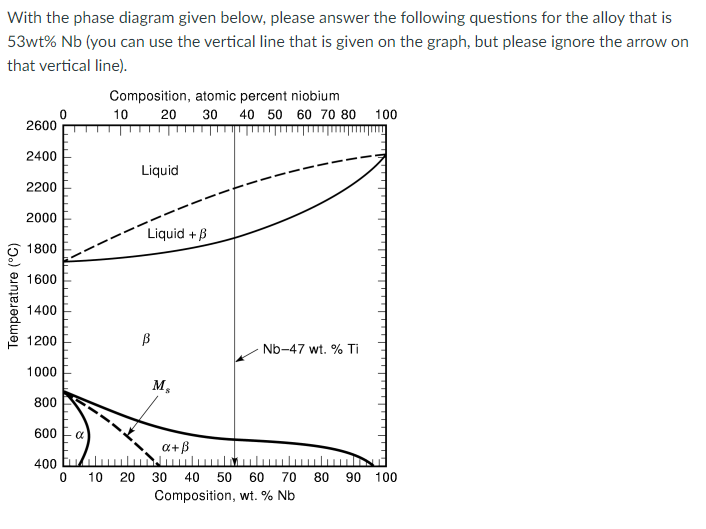
Q1. To heat up a solid alloy, at what temperature does melting start to occur?
Q2. What is the composition of the first liquid phase when heating the alloy up from room temperature?
Q3.To solidify the liquid solution from 2400 C, at what temperature does solidification start to occur?
Q4.What is(are) the composition(s) of each phase(s) at 2400C?
Q5.What is(are) the mass fraction of each phase at 2400 C?
Q6.What phase(s) present(s) at 2000 C?
Q7.What is(are) the composition(s) of each phase(s) at 2000 C?
Q8.What phase(s) present(s) at 2400 C?
With the phase diagram given below, please answer the following questions for the alloy that is 53wt% Nb (you can use the vertical line that is given on the graph, but please ignore the arrow on that vertical line). Composition, atomic percent niobium 10 20 30 40 50 60 70 80 2600 100 2400 Liquid 2200 2000 Liquid +B 1800 1600 Temperature (C) 1400 1200 B Nb-47 wt. % Ti 1000 M 800 600 a+B 400 tu 0 10 20 80 90 100 30 40 50 60 70 Composition, wt. % Nb
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


