Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q1. Two possible designs are being considered for an irreversible elementary liquid phase reaction: A (aq) +B (aq) C (aq) + D (g) The
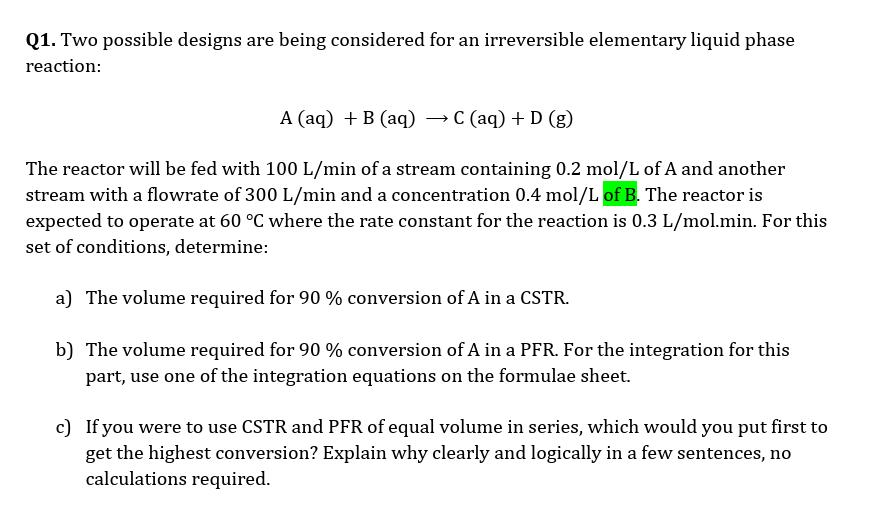
Q1. Two possible designs are being considered for an irreversible elementary liquid phase reaction: A (aq) +B (aq) C (aq) + D (g) The reactor will be fed with 100 L/min of a stream containing 0.2 mol/L of A and another stream with a flowrate of 300 L/min and a concentration 0.4 mol/L of B. The reactor is expected to operate at 60 C where the rate constant for the reaction is 0.3 L/mol.min. For this set of conditions, determine: a) The volume required for 90 % conversion of A in a CSTR. b) The volume required for 90 % conversion of A in a PFR. For the integration for this part, use one of the integration equations on the formulae sheet. c) If you were to use CSTR and PFR of equal volume in series, which would you put first to get the highest conversion? Explain why clearly and logically in a few sentences, no calculations required.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


