Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q10- A 20mL vinegar sample (d=1.0g/mL, molar mass of acetic acid =60.0g/mol) has been titrated with a 1.2MNaOH solution, If the initial reading of the
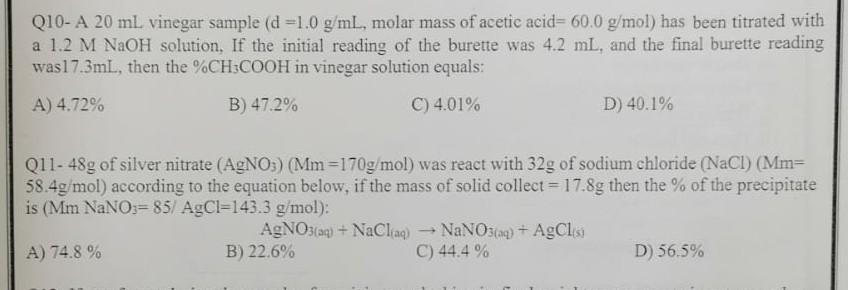
Q10- A 20mL vinegar sample (d=1.0g/mL, molar mass of acetic acid =60.0g/mol) has been titrated with a 1.2MNaOH solution, If the initial reading of the burette was 4.2mL, and the final burette reading was 17.3mL, then the %CH3COOH in vinegar solution equals: A) 4.72% B) 47.2% C) 4.01% D) 40.1% Q11- 48g of silver nitrate (AgNO3)(Mm=170g/mol) was react with 32g of sodium chloride (NaCl)(Mm= 58.4g/mol ) according to the equation below, if the mass of solid collect =17.8g then the % of the precipitate is (MmNaNO=85/AgCl3=143.3g/mol) : A) 74.8% B) 22.6% C) 44.4% D) 56.5%
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


