Question
Q-13. Which of the presented below formulas don't present the Nernst equation? a) Ecell = Ecell RT/nF (In Q); b) Ecell = Ecell- 0.0592/n
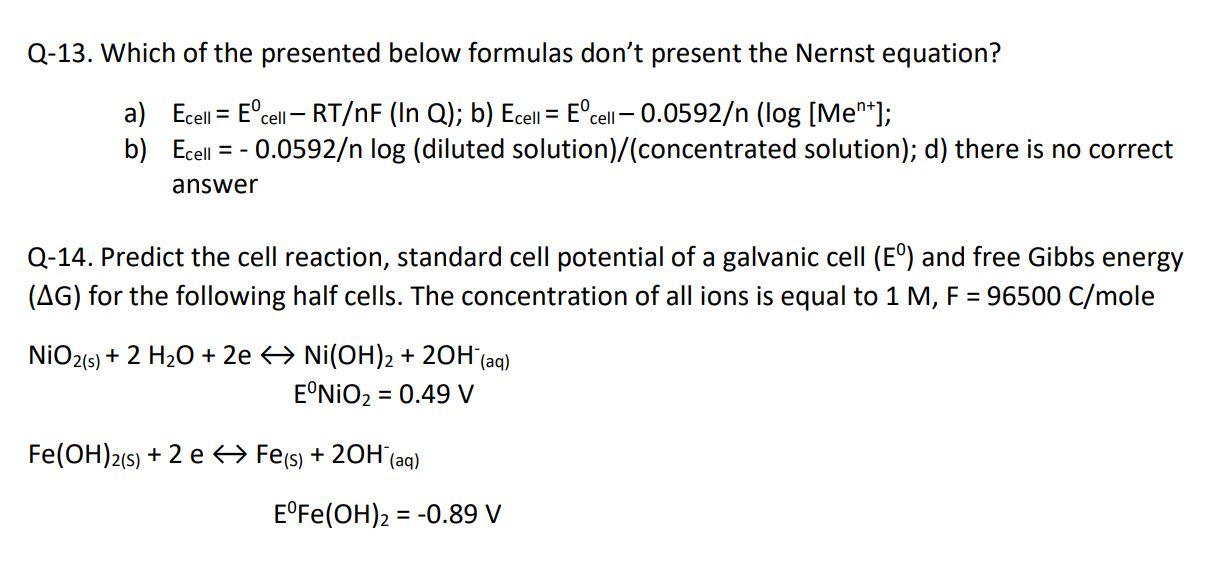
Q-13. Which of the presented below formulas don't present the Nernst equation? a) Ecell = Ecell RT/nF (In Q); b) Ecell = Ecell- 0.0592/n (log [Me*]; b) Ecell = - 0.0592/n log (diluted solution)/(concentrated solution); d) there is no correct answer Q-14. Predict the cell reaction, standard cell potential of a galvanic cell (E) and free Gibbs energy (AG) for the following half cells. The concentration of all ions is equal to 1 M, F = 96500 C/mole NIO2(s) + 2 H20 + 2e > Ni(OH)2 + 20H (aq) ENIO2 = 0.49 V Fe(OH)2(s) + 2 e Fe(s) + 20H(aq) EFe(OH)2 = -0.89 V
Step by Step Solution
3.45 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
Ques 13 answ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of biochemistry Life at the Molecular Level
Authors: Donald Voet, Judith G. Voet, Charlotte W. Pratt
4th edition
470547847, 978-0470547847
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



