Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q13,12 You need to make an aqueous solution of 0.239M magnesium sulfate for an experiment in lab, using a 250m volumetric flask. How much solid
Q13,12 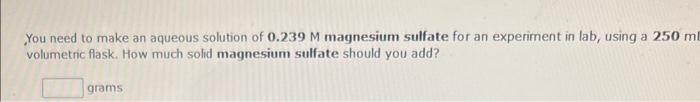

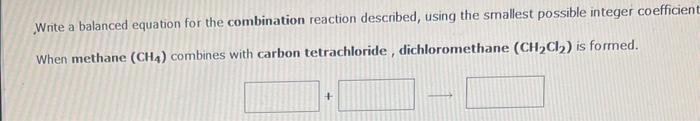

You need to make an aqueous solution of 0.239M magnesium sulfate for an experiment in lab, using a 250m volumetric flask. How much solid magnesium sulfate should you add? grams: How many millititers of an aqueous solution of 0.205M potassium nitrate is needed to obtain 14.9 grams of the salt mL Write a balanced equation for the combination reaction described, using the smallest possible integer coefficien When methane (CH4) combines with carbon tetrachloride, dichloromethane (CH2Cl2) is formed. Write a balanced equation for the combination reaction described, using the smallest possible integer coefficients. When zinc oxide combines with water, zinc hydroxide is formed 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


