Question
Balance each of the following reaction equations. If no coefficient is needed, leave the line blank. 1) CH2(g) + H2(g) CH6(8) 2) C(s) +
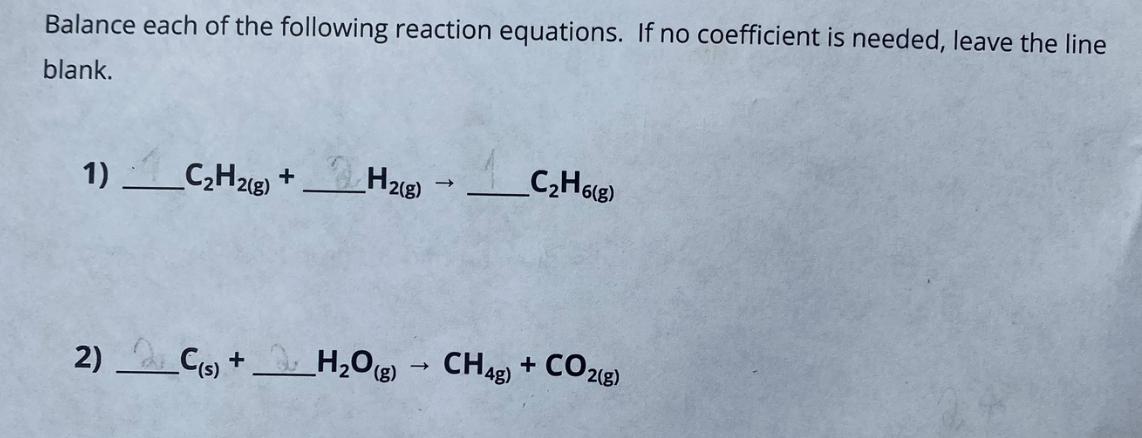
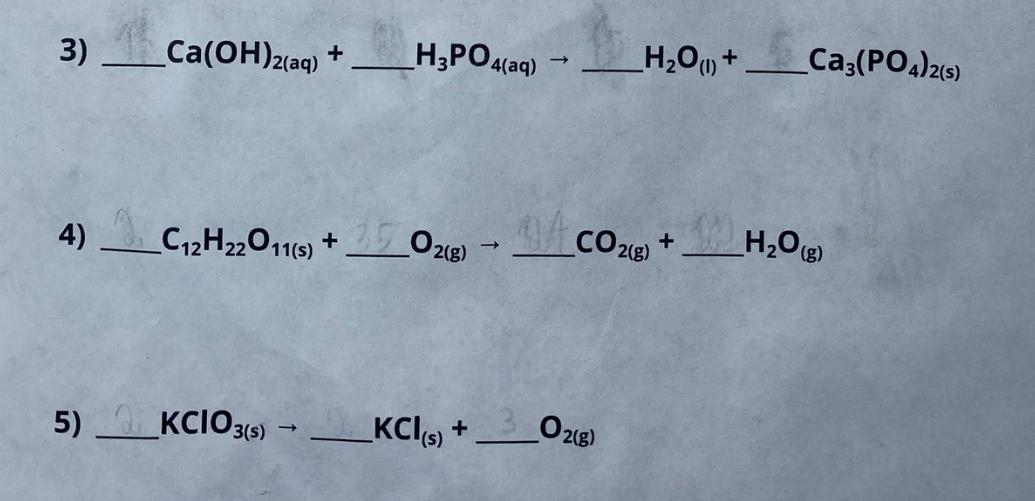
Balance each of the following reaction equations. If no coefficient is needed, leave the line blank. 1) CH2(g) + H2(g) CH6(8) 2) C(s) + H2O(g) CH4g) + CO2(g) 3) Ca(OH)2(aq) + H3PO4(aq) -> H2O(l) + Ca3(PO4)2(s) 4) C12H22O11(s) + O2(g) -> CO2(g) + HO(g) 5) KCIO3(s) KCl (s) + O2(g)
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Chemical equations represent the reactants and products involved in ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of Ethics for Scientists and Engineers
Authors: Edmund G. Seebauer, Robert L. Barry
1st Edition
9780195698480, 195134885, 195698487, 978-0195134889
Students also viewed these Programming questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



