Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q2 (20 points). Entropy change a) Consider a thermally isolated flow chamber of volume V=L3 as shown above. On the surface of this chamber, there
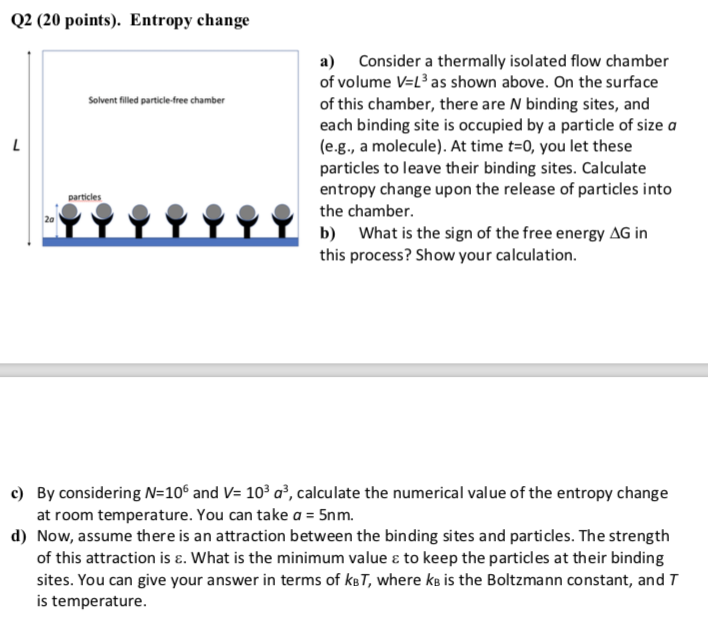 Q2 (20 points). Entropy change a) Consider a thermally isolated flow chamber of volume V=L3 as shown above. On the surface of this chamber, there are N binding sites, and each binding site is occupied by a particle of size a (e.g., a molecule). At time t=0, you let these particles to leave their binding sites. Calculate entropy change upon the release of particles into the chamber. b) What is the sign of the free energy G in this process? Show your calculation. c) By considering N=106 and V=103a3, calculate the numerical value of the entropy change at room temperature. You can take a=5nm. d) Now, assume there is an attraction between the binding sites and particles. The strength of this attraction is . What is the minimum value to keep the particles at their binding sites. You can give your answer in terms of kBT, where kB is the Boltzmann constant, and T is temperature
Q2 (20 points). Entropy change a) Consider a thermally isolated flow chamber of volume V=L3 as shown above. On the surface of this chamber, there are N binding sites, and each binding site is occupied by a particle of size a (e.g., a molecule). At time t=0, you let these particles to leave their binding sites. Calculate entropy change upon the release of particles into the chamber. b) What is the sign of the free energy G in this process? Show your calculation. c) By considering N=106 and V=103a3, calculate the numerical value of the entropy change at room temperature. You can take a=5nm. d) Now, assume there is an attraction between the binding sites and particles. The strength of this attraction is . What is the minimum value to keep the particles at their binding sites. You can give your answer in terms of kBT, where kB is the Boltzmann constant, and T is temperature Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


