Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q.2. (8 marks) Consider the process shown in the figure below, where carbon monoxide (CO) gas is being oxidized to carbon dioxide (CO2). This process
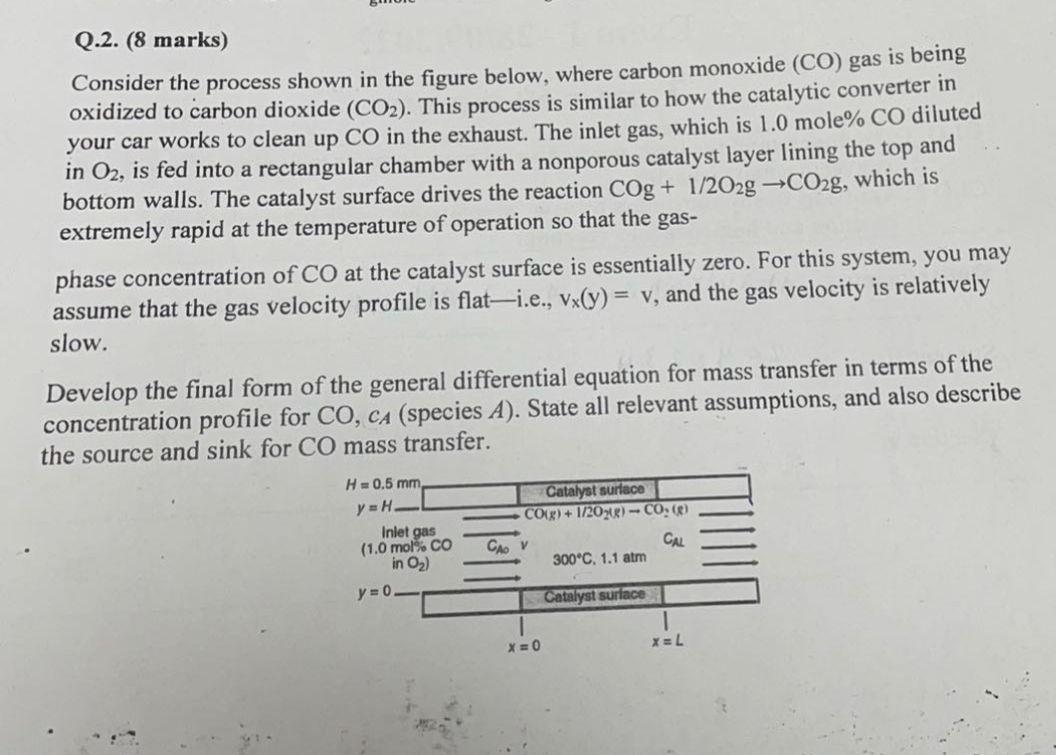
Q.2. (8 marks) Consider the process shown in the figure below, where carbon monoxide (CO) gas is being oxidized to carbon dioxide (CO2). This process is similar to how the catalytic converter in your car works to clean up CO in the exhaust. The inlet gas, which is 1.0mole%CO diluted in O2, is fed into a rectangular chamber with a nonporous catalyst layer lining the top and bottom walls. The catalyst surface drives the reaction COg+1/2O2gCO2g, which is extremely rapid at the temperature of operation so that the gas- phase concentration of CO at the catalyst surface is essentially zero. For this system, you may assume that the gas velocity profile is flat-i.e., vx(y)=vy, and the gas velocity is relatively slow. Develop the final form of the general differential equation for mass transfer in terms of the concentration profile for CO,cA (species A ). State all relevant assumptions, and also describe the source and sink for CO mass transfer
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


