Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q2. Determine the calcination temperature for MgCO3 a) In the environment with composition of 30% CO,and 70% N2 at total 1 atmpressure, b) In
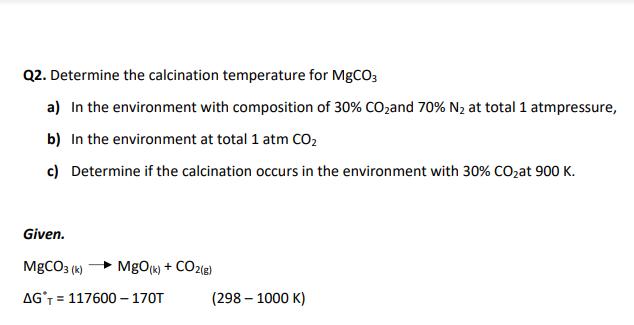
Q2. Determine the calcination temperature for MgCO3 a) In the environment with composition of 30% CO,and 70% N2 at total 1 atmpressure, b) In the environment at total 1 atm CO2 c) Determine if the calcination occurs in the environment with 30% C02at 900 K. Given. MgCO3 (k) MgO(k) + CO2(8) AG'T = 117600 170T (298 1000 K)
Step by Step Solution
★★★★★
3.43 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
The formula used is G G RT ln k p Since this is a forward direction reaction so G will be le...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


