Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q2 The isobaric, isothermal, gas-phase decomposition of dimethyl ether (CH3OCH3) at 800K produces methane gas (CH4), hydrogen gas (H2) and carbon monoxide gas (CO). The
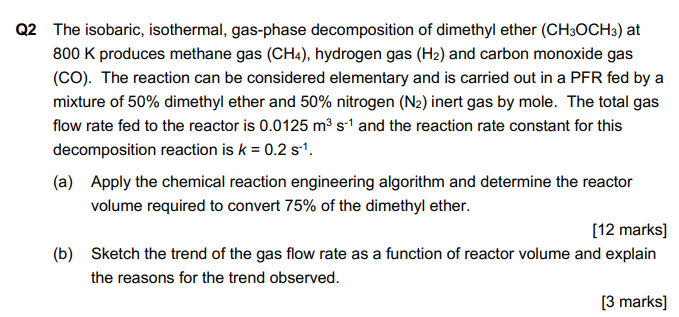 Q2 The isobaric, isothermal, gas-phase decomposition of dimethyl ether (CH3OCH3) at 800K produces methane gas (CH4), hydrogen gas (H2) and carbon monoxide gas (CO). The reaction can be considered elementary and is carried out in a PFR fed by a mixture of 50% dimethyl ether and 50% nitrogen (N2) inert gas by mole. The total gas flow rate fed to the reactor is 0.0125m3s1 and the reaction rate constant for this decomposition reaction is k=0.2s1. (a) Apply the chemical reaction engineering algorithm and determine the reactor volume required to convert 75% of the dimethyl ether. [12 marks] (b) Sketch the trend of the gas flow rate as a function of reactor volume and explain the reasons for the trend observed
Q2 The isobaric, isothermal, gas-phase decomposition of dimethyl ether (CH3OCH3) at 800K produces methane gas (CH4), hydrogen gas (H2) and carbon monoxide gas (CO). The reaction can be considered elementary and is carried out in a PFR fed by a mixture of 50% dimethyl ether and 50% nitrogen (N2) inert gas by mole. The total gas flow rate fed to the reactor is 0.0125m3s1 and the reaction rate constant for this decomposition reaction is k=0.2s1. (a) Apply the chemical reaction engineering algorithm and determine the reactor volume required to convert 75% of the dimethyl ether. [12 marks] (b) Sketch the trend of the gas flow rate as a function of reactor volume and explain the reasons for the trend observed Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


