Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q3 At low to moderate pressures, the equilibrium state of the water-gas shift reaction performs as below: CO+H,0 C02 + H2 is approximately described by
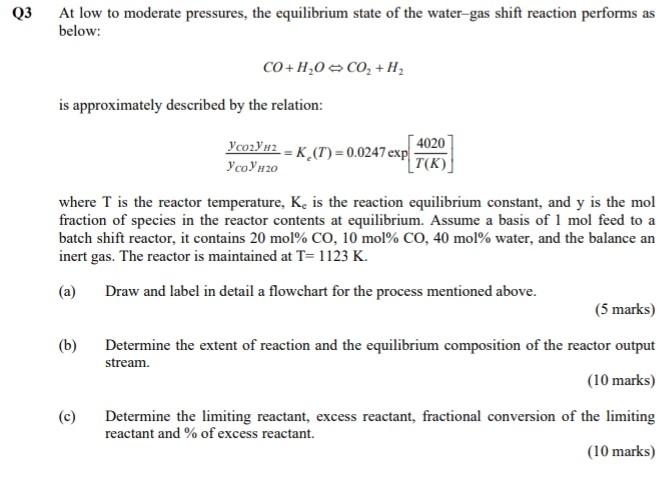
Q3 At low to moderate pressures, the equilibrium state of the water-gas shift reaction performs as below: CO+H,0 C02 + H2 is approximately described by the relation: Ycozy #2 = K (T) = 0.0247 exp 4020 y co 20 T(K) where T is the reactor temperature, K. is the reaction equilibrium constant, and y is the mol fraction of species in the reactor contents at equilibrium. Assume a basis of 1 mol feed to a batch shift reactor, it contains 20 mol% CO, 10 mol% CO, 40 mol% water, and the balance an inert gas. The reactor is maintained at T=1123 K. (a) Draw and label in detail a flowchart for the process mentioned above. (5 marks) (b) Determine the extent of reaction and the equilibrium composition of the reactor output stream. (10 marks) (c) Determine the limiting reactant, excess reactant, fractional conversion of the limiting reactant and % of excess reactant. (10 marks)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


