Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q3: Refer to the reactor system shown in Fig. 2. The reaction is exothermic. A cooling system is provided to remove the excess energy
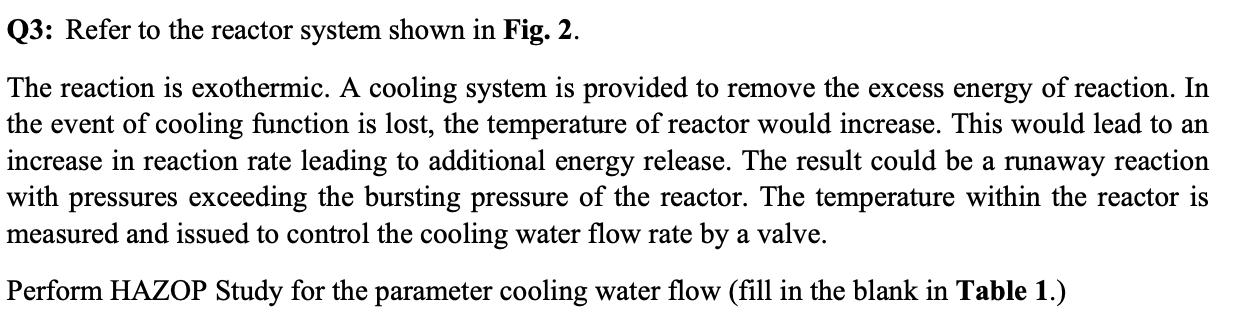
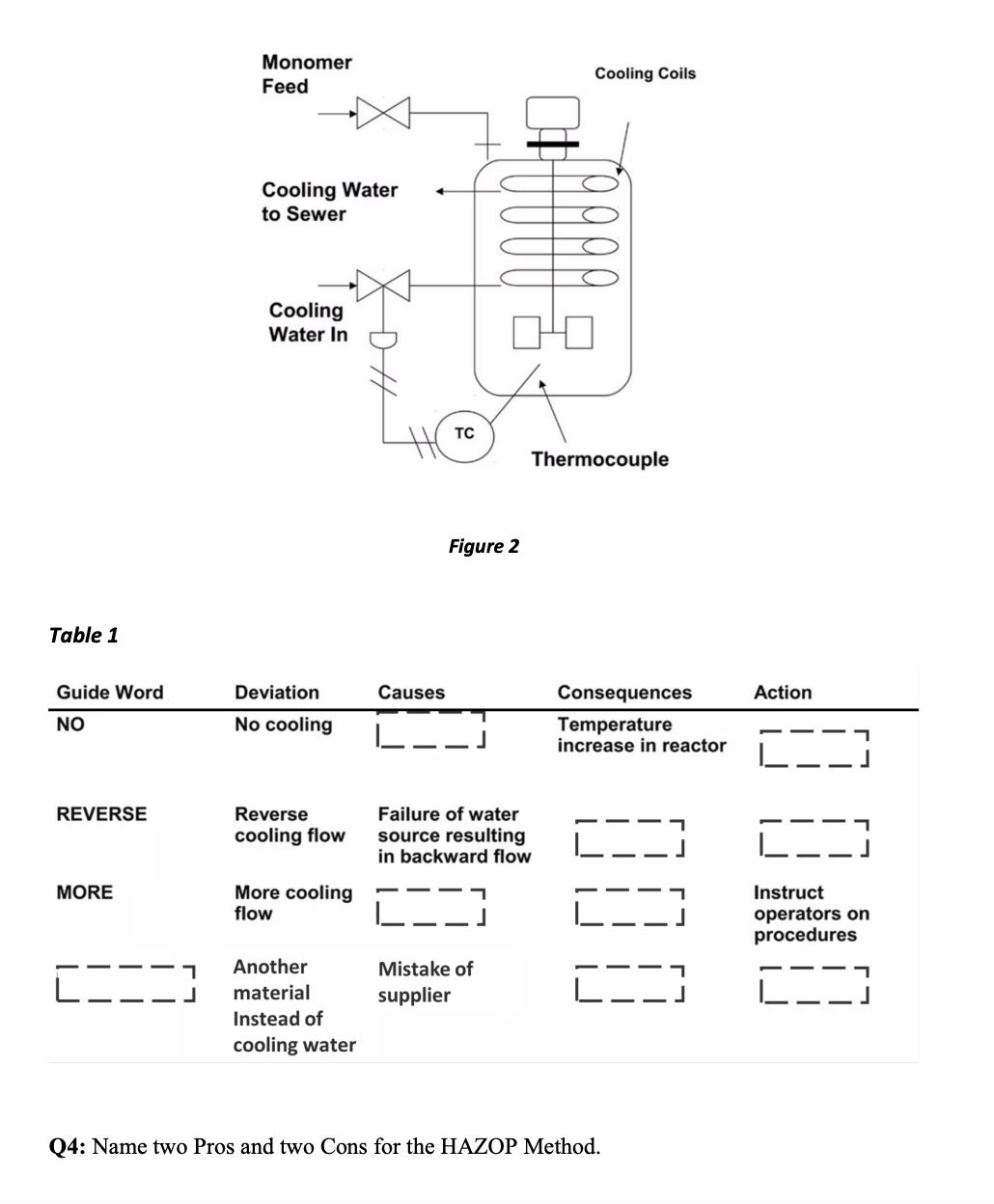
Q3: Refer to the reactor system shown in Fig. 2. The reaction is exothermic. A cooling system is provided to remove the excess energy of reaction. In the event of cooling function is lost, the temperature of reactor would increase. This would lead to an increase in reaction rate leading to additional energy release. The result could be a runaway reaction with pressures exceeding the bursting pressure of the reactor. The temperature within the reactor is measured and issued to control the cooling water flow rate by a valve. Perform HAZOP Study for the parameter cooling water flow (fill in the blank in Table 1.) Table 1 Guide Word NO REVERSE MORE 1 J Monomer Feed Cooling Water to Sewer Cooling Water In Deviation No cooling Reverse cooling flow More cooling flow Another material Instead of cooling water Causes L. TC Figure 2 Failure of water source resulting in backward flow L-- 00 7 Mistake of supplier Cooling Coils Thermocouple Consequences Temperature increase in reactor Q4: Name two Pros and two Cons for the HAZOP Method. Action L Instruct operators on procedures
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


