Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q3 You are asked to produce phenol and acetone from benzene and propylene. Do the material balance for producing 5000 tons of phenol. Calculate Enhancement

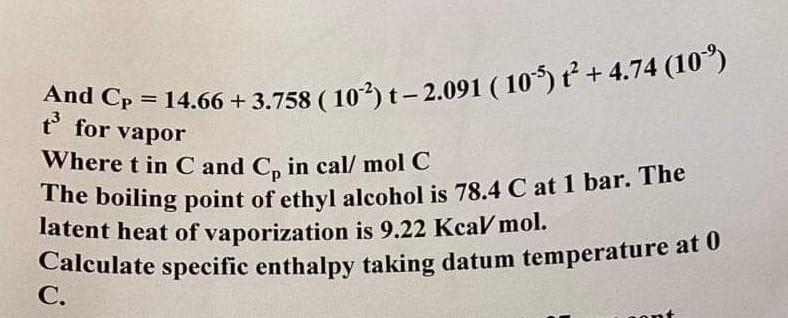
Q3 You are asked to produce phenol and acetone from benzene and propylene. Do the material balance for producing 5000 tons of phenol. Calculate Enhancement ratio. Substance Price $ ton Benzene 1300 Propylene 1378 Cumene 1480 Phenol 1650 Acetone 1200 Oxygen 500 Benzene +Propylene+ Oxygen---->Phenol+acetone C6H6 C3H6 02 C6H5OH C3H60 Show which is more economical to produce cumene or phenol and acetone. And Cp for vapor = 14.66 + 3.758 (10-2)t-2.091 ( 10-5 +4.74 (109) Where t in C and Co in cal/mol C The boiling point of ethyl alcohol is 78.4 C at 1 bar. The latent heat of vaporization is 9.22 Kca/mol. Calculate specific enthalpy taking datum temperature at 0 C
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


