Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q4 (30 pts). Consider the reversible reaction of hydrogen and iodine to form hydrogen iodide: H2(g)+I2(g)2HI(g) This reaction reaches equilibrium at a certain temperature (T),
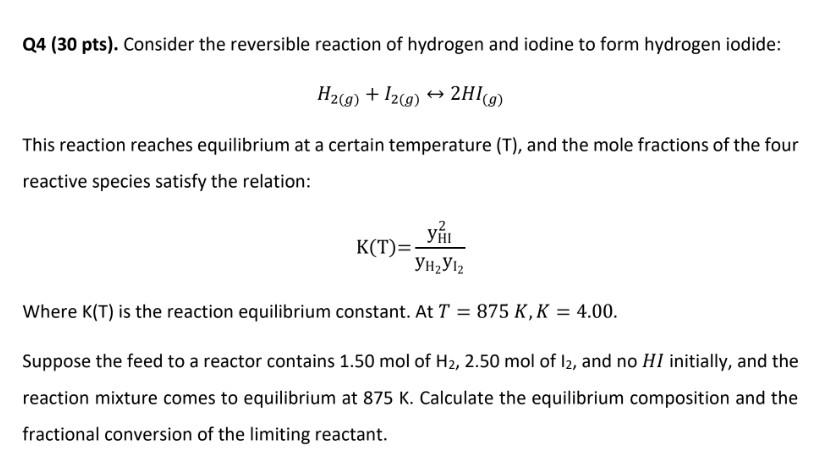
Q4 (30 pts). Consider the reversible reaction of hydrogen and iodine to form hydrogen iodide: H2(g)+I2(g)2HI(g) This reaction reaches equilibrium at a certain temperature (T), and the mole fractions of the four reactive species satisfy the relation: K(T)=yH2yI2yHI2 Where K(T) is the reaction equilibrium constant. At T=875K,K=4.00. Suppose the feed to a reactor contains 1.50molofH2,2.50mol of I2, and no HI initially, and the reaction mixture comes to equilibrium at 875K. Calculate the equilibrium composition and the fractional conversion of the limiting reactant
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


