Question
Q4. A co-ordination complex of platinum has an empirical formula PtCl3 (NH3)2(NO2). On reacting with excess silver nitrate, one mole of the complexes produces
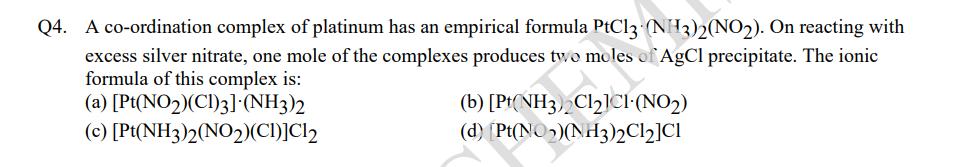
Q4. A co-ordination complex of platinum has an empirical formula PtCl3 (NH3)2(NO2). On reacting with excess silver nitrate, one mole of the complexes produces two moles of AgCl precipitate. The ionic formula of this complex is: (a) [Pt(NO2)(CI)3] (NH3)2 (c) [Pt(NH3)2(NO2)(CI)]C12 (b) [Pt(NH3)2ClICI (NO) (d) [Pt(NO)(NH3)2C12]C1
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Soluti...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles
Authors: Steven S. Zumdahl, Donald J. DeCoste
7th edition
9781133109235, 1111580650, 978-1111580650
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



