Question
Q.4 (a) For a reaction, A + B'n Product, the rate law is given by r = k [A] [B]* . What is 1/2
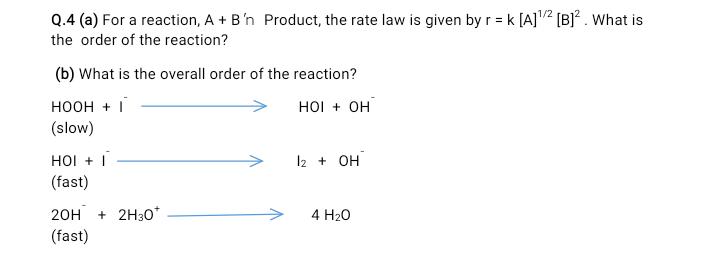
Q.4 (a) For a reaction, A + B'n Product, the rate law is given by r = k [A] [B]* . What is 1/2 the order of the reaction? (b) What is the overall order of the reaction? HOOH + I HOI + OH (slow) HI + | 12 + OH (fast) 20H + 2H30* 4 H20 (fast)
Step by Step Solution
3.45 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
Overall order of rea...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Microeconomics
Authors: Robert Pindyck, Daniel Rubinfeld
8th edition
978-0132870436, 132870436, 013285712X, 978-0133371178, 133371174, 978-0132857123
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



