Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q4 A stream of air flowing at a rate of 10 kg/h at STP (1 mol = 22.4 m? (STP)/kmol) is mixed with a stream
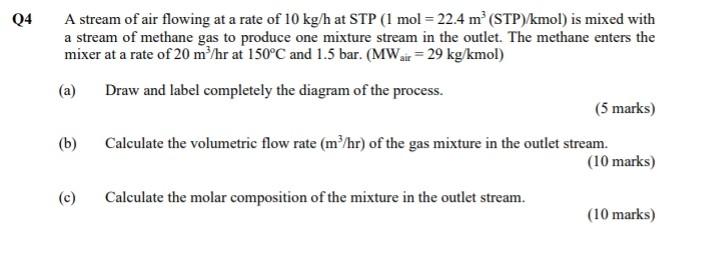
Q4 A stream of air flowing at a rate of 10 kg/h at STP (1 mol = 22.4 m? (STP)/kmol) is mixed with a stream of methane gas to produce one mixture stream in the outlet. The methane enters the mixer at a rate of 20 m/hr at 150C and 1.5 bar. (MWar = 29 kg/kmol) (a) Draw and label completely the diagram of the process. (5 marks) Calculate the volumetric flow rate (m/hr) of the gas mixture in the outlet stream. (10 marks) (c) Calculate the molar composition of the mixture in the outlet stream. (10 marks) (b)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


