Question: Q.5 please help with the data treatment! Introduction This experiment makes use of the fluoride ion-selective electrode to determine the fluoride concentration in commercial anti-plaque


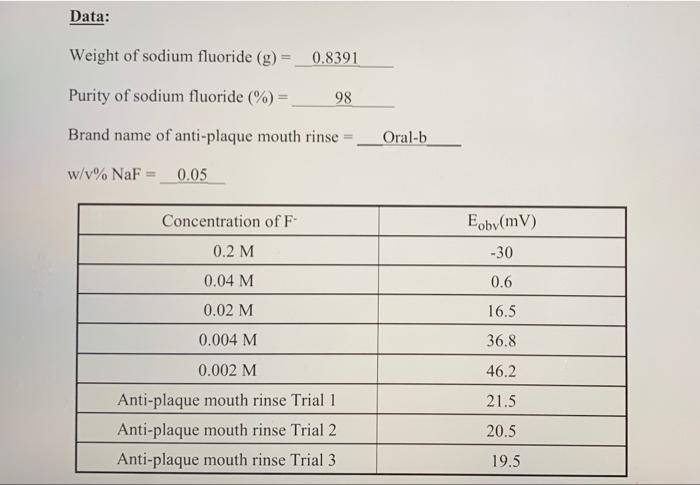
Introduction This experiment makes use of the fluoride ion-selective electrode to determine the fluoride concentration in commercial anti-plaque mouth rinse and toothpaste. The fluoride ion-selective electrode is a typical solid state type electrode which ideally gives a Nernstian response towards fluoride ion in solution. The technique is used to determine fluoride contents in tap water, commercial toothpastes and anti-plaque mouth rinse in the market. Lanthanum fluoride, LaFs, is a nearly ideal substance for the preparation of a crystalline membrane electrode for the determination of fluoride ion. Although this compound is a natural conductor, its conductivity can be enhanced by doping with europium fluoride, EuF2. Membranes are prepared by cutting disks from a single crystal of the doped compound. The mechanism of the development of a fluoride sensitive potential across a lanthanum fluoride membrane is quite analogous to that described for glass, pH-sensitive membranes. That is, at the two interfaces, ionization creates a charge on the membrane surface as shown by the equation LaF; LaFy* + F solid solid solution The magnitude of the charge is dependent upon the fluoride ion concentration of the solution. Thus, the side of the membrane in contact with the solution of lower fluoride ion concentration becomes positive with respect to the other surface; it is this charge difference that provides a measure of the difference in fluoride concentration of the two solutions. The potential of a cell containing a lanthanum fluoride electrode is given by the following equation: 0.0592 E = K - -log[F] E. = measured potential K= constant n= charge on the fluoride ion F = concentration of unknown fluoride solution Thus if the potentials of a set of standards of known concentration are measured, a plot of potential, E. vs. log concentration should give a straight line whose slope should approximate -0.0592 volts/unit charge, remembering that the equation holds at 25C. n un a a 25 Procedure A. Preparation of the Buffer solution 1. Weigh out 10.0 g of sodium acetate and 14.5 g of sodium chloride, dissolves them in 100 mL deionized water. 2. Then add 7.5 mL of acetic acid make up to 250 mL of solution in a volumetric flask. B. Preparation of the Fluoride Calibration Curve 1. Weight 0.8398 g NaF and dissolve in 50 mL DI water and dilute to the mark of 100 mL volumetric flask as 0.2 M NaF solution. 2. Then prepare 4 x 10-2, 2 x 10-2,4 x 10-3,2 x 10-3 M NaF solution from 0.2 M NaF solution using 100 mL volumetric flask. 3. Pipet 10 ml of each NaF solution and buffer solution in dried 100 mLbeakers. Then measure the potential difference by a millivolt meter using fluoride selective electrode. C. Sample Measurement 1. Pipet 10 mL of the sample provided and 10 mL buffer in 100 mL dry beakers and measure the Eoby for the sample solution. Repeat two more times. Data Treatment 1. Plot a correlation of Eoby versus log [NaF) and obtain the calibration curve by using the least square method. 2. Calculate the sodium fluoride content (% w/v) in the Anti-Plaque Mouth rinse sample. 3. Compare and account for the differences between the labeled composition and your results. Questions 1. What is the purpose of the buffer and why is it necessary? Data: Weight of sodium fluoride (g) = 0.8391 Purity of sodium fluoride (%) = 98 Brand name of anti-plaque mouth rinse Oral-b w/v% NaF = 0.05 Eoby(mV) Concentration of F- 0.2 M 0.04 M -30 0.6 16.5 36.8 46.2 0.02 M 0.004 M 0.002 M Anti-plaque mouth rinse Trial 1 Anti-plaque mouth rinse Trial 2 Anti-plaque mouth rinse Trial 3 21.5 20.5 19.5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


