Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Veff (r) (eV) The following figure plots potential energy as a function of distance from the nucleus for three different electrons in a sodium
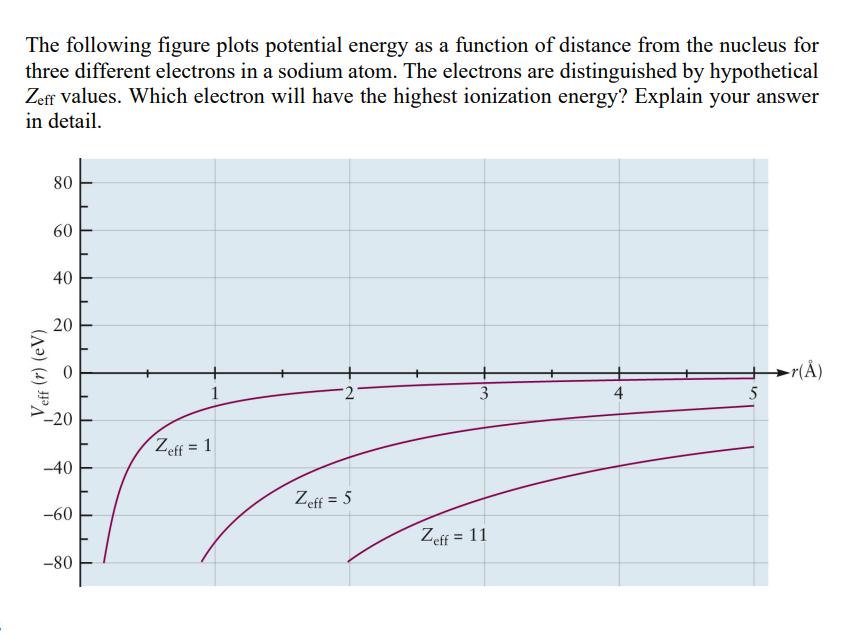
Veff (r) (eV) The following figure plots potential energy as a function of distance from the nucleus for three different electrons in a sodium atom. The electrons are distinguished by hypothetical Zeff values. Which electron will have the highest ionization energy? Explain your answer in detail. 80 80 60 40 20 20 -20 Zeff = 1 -40 -60 -80 1 2' 3 4 5 Zeff = 5 Zeff = 11 r()
Step by Step Solution
★★★★★
3.42 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
Solutions Step 1 Explanation how to determine which electron has the highest ionization energy based on the effective nuclear charge Zeff Ionization e...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


