Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q1) The vapour pressure, p, of nitric acid varies with temperature as follows: 0/C 0 20 40 50 70 80 90 100 p/kPa 1.92
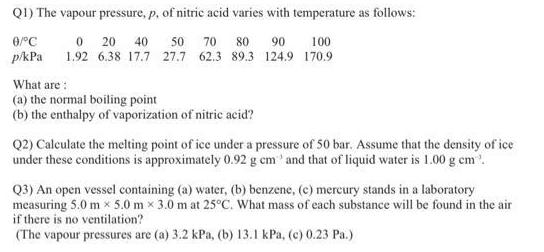
Q1) The vapour pressure, p, of nitric acid varies with temperature as follows: 0/C 0 20 40 50 70 80 90 100 p/kPa 1.92 6.38 17.7 27.7 62.3 89.3 124.9 170.9 What are: (a) the normal boiling point (b) the enthalpy of vaporization of nitric acid? Q2) Calculate the melting point of ice under a pressure of 50 bar. Assume that the density of ice under these conditions is approximately 0.92 g cm' and that of liquid water is 1.00 g cm. Q3) An open vessel containing (a) water, (b) benzene, (c) mercury stands in a laboratory measuring 5.0 m 5.0 mx 3.0 m at 25C. What mass of each substance will be found in the air if there is no ventilation? (The vapour pressures are (a) 3.2 kPa, (b) 13.1 kPa, (c) 0.23 Pa.)
Step by Step Solution
★★★★★
3.46 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
1a We have HNO3 and we have to determine its boiling point and enthalpy of vaporization We know that d ln p dt vap H RT2 d ln p vap H RT2 dT d ln p va...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


