Answered step by step
Verified Expert Solution
Question
1 Approved Answer
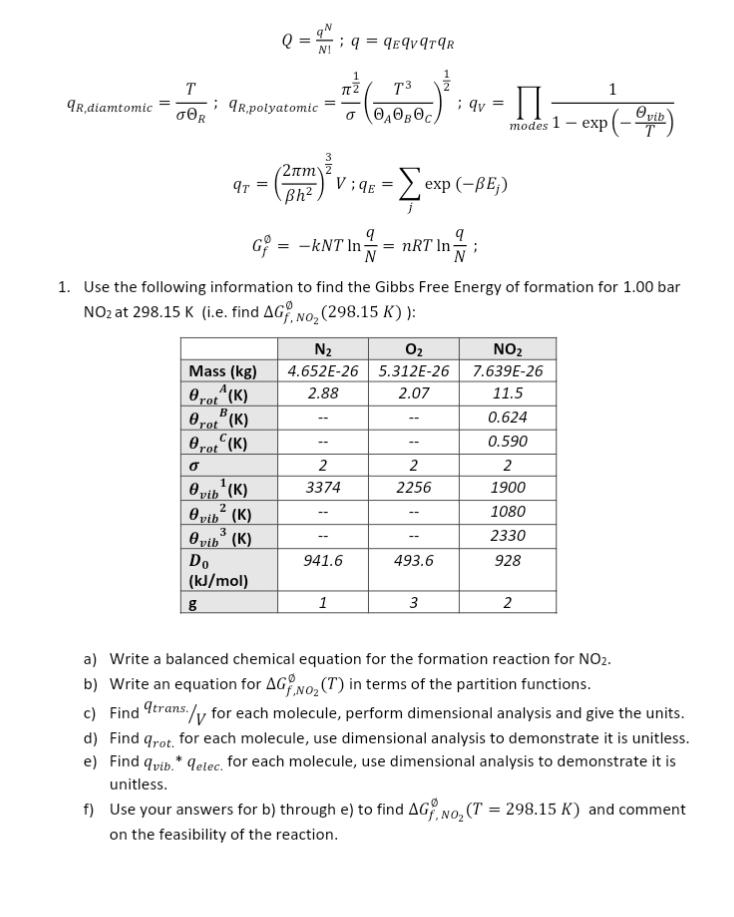
Q=N!qN;q=qEqVqTqRqR,diamtomic=RT;qR,polyatomic=21(ABCT3)21;qV=modes1exp(TVib)1qT=(h22m)23V;qE=jexp(Ej)Gf=kNTlnNq=nRTlnNq; 1. Use the following information to find the Gibbs Free Energy of formation for 1.00 bar NO2 at 298.15K (i.e. find Gf.NO2(298.15K) ): a)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


