Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Qt-11: Calculate the gross and net calorific value in kJ/kg for a coal which analyses: C:74%,H : 6%,N:1%,0:9%, S: 0.8%, moisture: 2.2% and ash 8%.
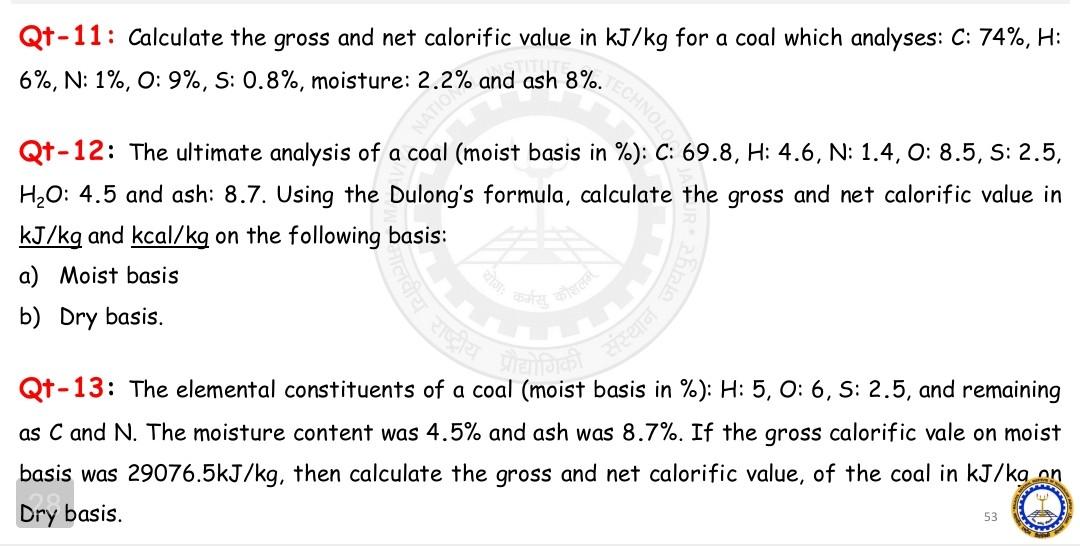
Qt-11: Calculate the gross and net calorific value in kJ/kg for a coal which analyses: C:74%,H : 6%,N:1%,0:9%, S: 0.8%, moisture: 2.2% and ash 8%. Qt-12: The ultimate analysis of a coal (moist basis in \%): C:69.8,H:4.6,N:1.4,0:8.5,S:2.5, H2O:4.5 and ash: 8.7. Using the Dulong's formula, calculate the gross and net calorific value in kJ/kg and kcal/kg on the following basis: a) Moist basis b) Dry basis. Qt-13: The elemental constituents of a coal (moist basis in \%): H: 5, O:6, S: 2.5, and remaining as C and N. The moisture content was 4.5% and ash was 8.7%. If the gross calorific vale on moist basis was 29076.5kJ/kg, then calculate the gross and net calorific value, of the coal in kJ/kann Dry basis
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


