Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Qt-21: Determine the air-fuel ratio and the theoretical amount of air required (mass basis) complete combustion of fuel containing 85% Carbon, 8% Hydrogen, 3% Oxygen,
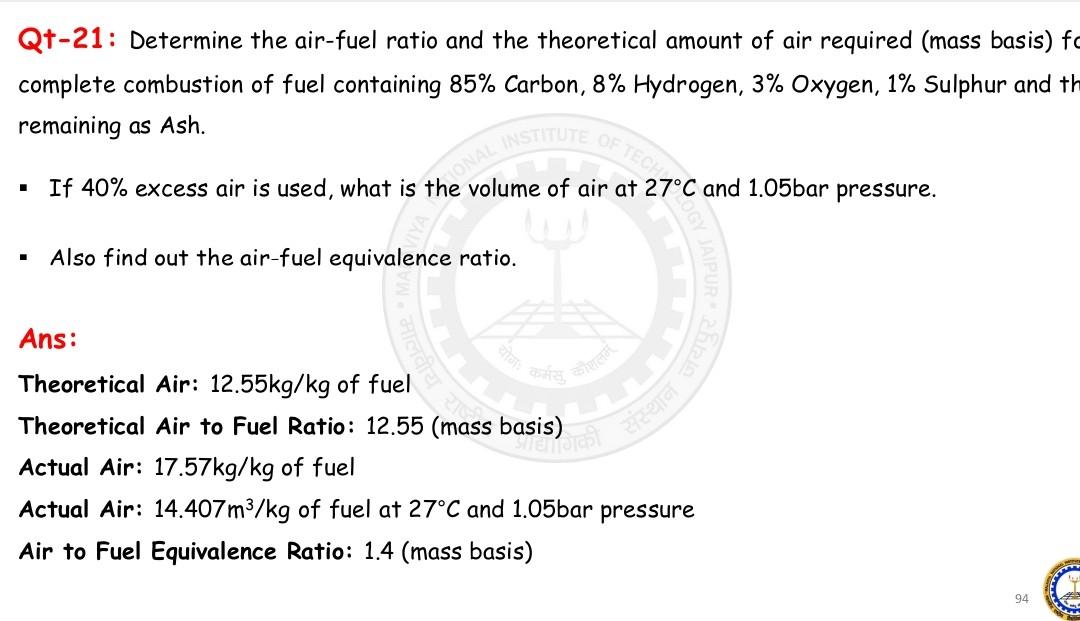
Qt-21: Determine the air-fuel ratio and the theoretical amount of air required (mass basis) complete combustion of fuel containing 85% Carbon, 8% Hydrogen, 3\% Oxygen, 1% Sulphur and remaining as Ash. - If 40% excess air is used, what is the volume of air at 27C and 1.05bar pressure. - Also find out the air-fuel equivalence ratio. Ans: Theoretical Air: 12.55kg/kg of fuel Theoretical Air to Fuel Ratio: 12.55 (mass basis) Actual Air: 17.57kg/kg of fuel Actual Air: 14.407m3/kg of fuel at 27C and 1.05 bar pressure Air to Fuel Equivalence Ratio: 1.4 (mass basis)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


