Answered step by step
Verified Expert Solution
Question
1 Approved Answer
QUESTION 1 120 Marks Caleite is a carbonate mineral and the most stable polymorph of calcium carbonate (CaCO3), It is a very common and widespread
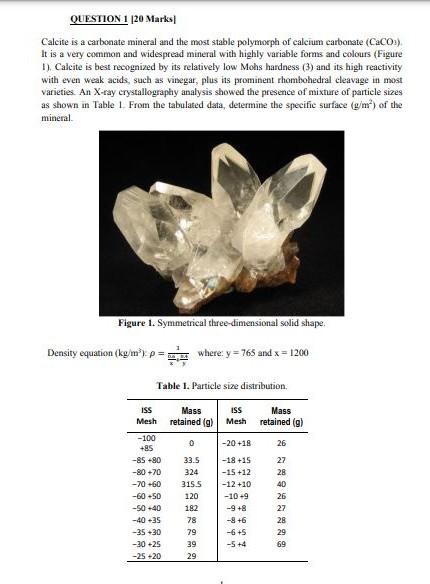
QUESTION 1 120 Marks Caleite is a carbonate mineral and the most stable polymorph of calcium carbonate (CaCO3), It is a very common and widespread mineral with highly variable forms and colours (Figure 1). Calcite is best recognized by its relatively low Mohs hardness (3) and its high reactivity with even weak acids, such as vinegar, plus its prominent rhombohedral cleavage in most varieties. An X-ray crystallography analysis showed the presence of mixture of particle sizes as shown in Table L. From the tabulated data, determine the specific surface (g/m) of the mineral Figure 1. Symmetrical three-dimensional solid shape = Density equation (kg n1, p=where : y = 765 and x = 1200 Table 1. Particle size distribution, ISS Mass retained (g) Mesh Mass retained (g) 0 -20 +18 26 33.5 324 ISS Mesh -100 +85 -85 +80 -80 +70 -70 -60 -60-50 -50 +40 -40 +35 -35-30 -30 +25 -25 +20 315.5 120 182 78 79 39 29 -18 +15 -15 +12 -12 +10 -10-9 -9 +8 -86 -6 +5 -5 +4 % l S E 6 b S = 27 28 40 26 27 28 29
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


