Answered step by step
Verified Expert Solution
Question
1 Approved Answer
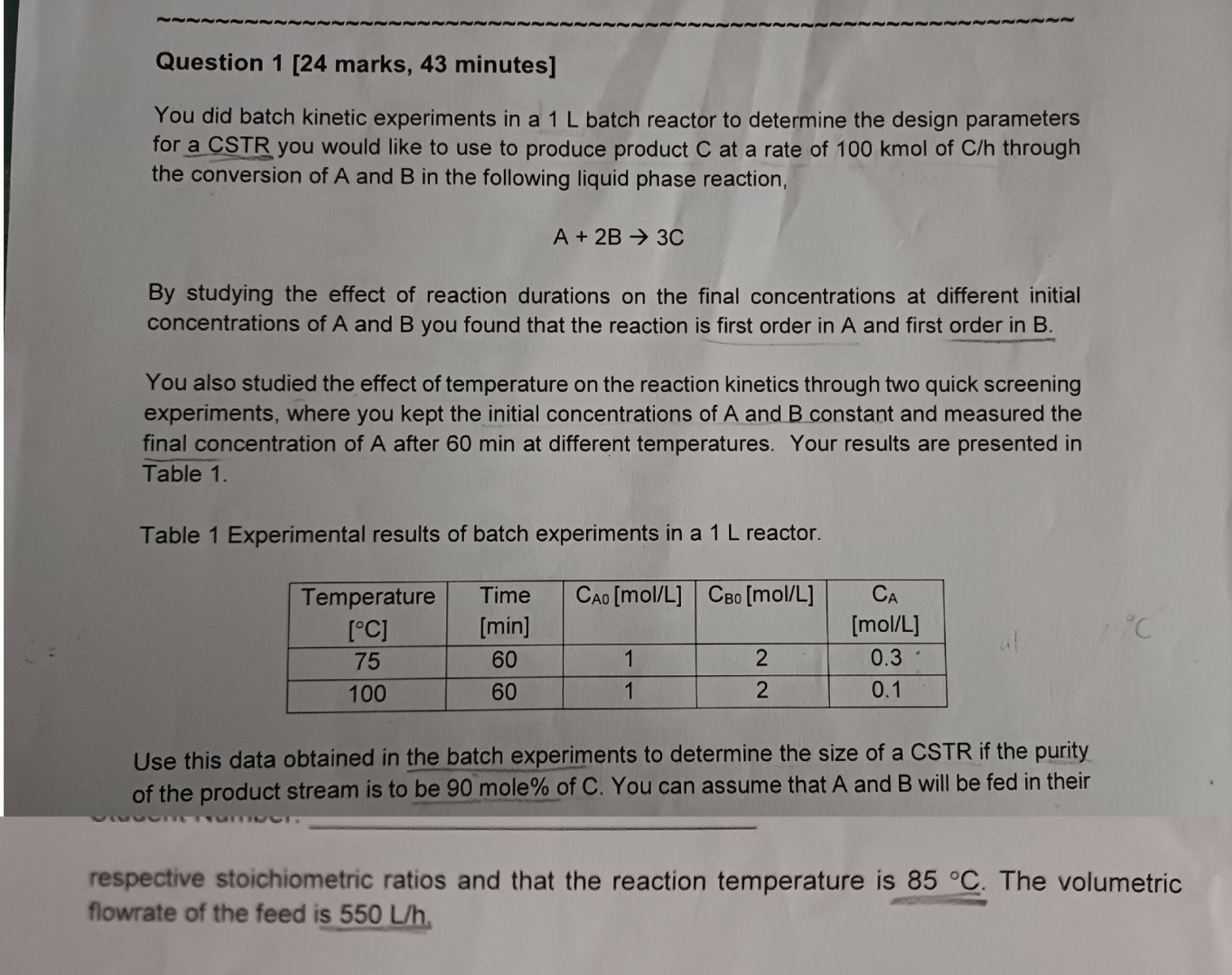
Question 1 [ 2 4 marks, 4 3 minutes ] You did batch kinetic experiments in a 1 L batch reactor to determine the design
Question marks, minutes
You did batch kinetic experiments in a batch reactor to determine the design parameters for a CSTR you would like to use to produce product at a rate of kmol of through the conversion of A and in the following liquid phase reaction,
By studying the effect of reaction durations on the final concentrations at different initial concentrations of A and you found that the reaction is first order in A and first order in
You also studied the effect of temperature on the reaction kinetics through two quick screening experiments, where you kept the initial concentrations of A and constant and measured the final concentration of A after min at different temperatures. Your results are presented in Table
Table Experimental results of batch experiments in a reactor.
tabletableTemperature
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


