Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A four-components gas mixture comprising of 44.7 kPa methane (CH4), 57 kPa ethane (C2H6), 60.3 kPa propane (C3H8), and 39.7 kPa n-butane (C4H10) flows
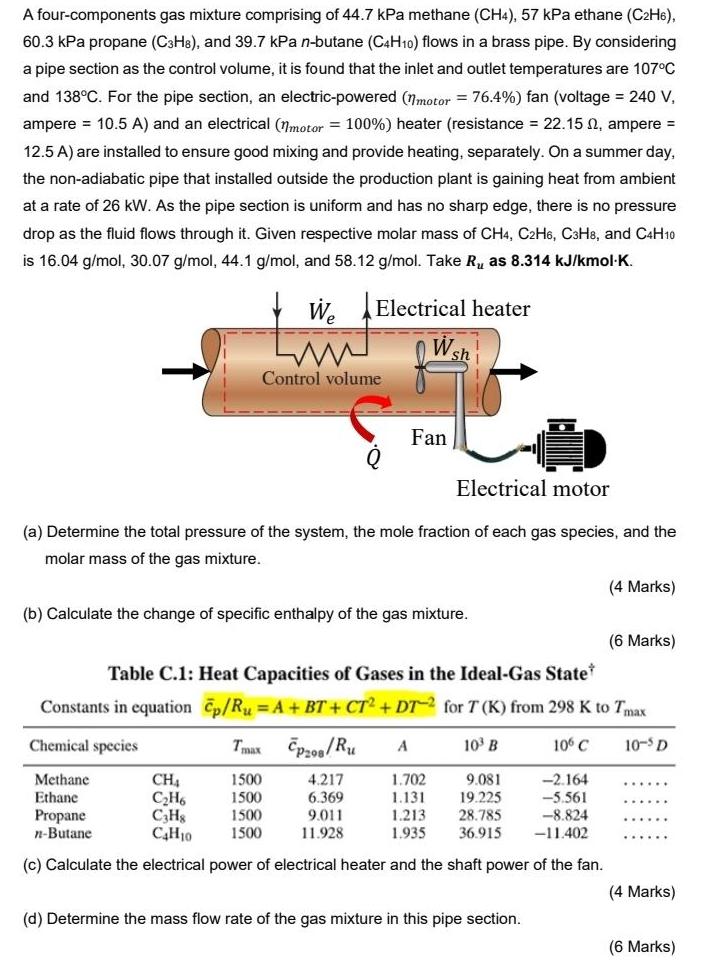
A four-components gas mixture comprising of 44.7 kPa methane (CH4), 57 kPa ethane (C2H6), 60.3 kPa propane (C3H8), and 39.7 kPa n-butane (C4H10) flows in a brass pipe. By considering a pipe section as the control volume, it is found that the inlet and outlet temperatures are 107C and 138C. For the pipe section, an electric-powered (nmotor = 76.4%) fan (voltage = 240 V, ampere = 10.5 A) and an electrical (7motor = 100%) heater (resistance = 22.15 N, ampere = 12.5 A) are installed to ensure good mixing and provide heating, separately. On a summer day, the non-adiabatic pipe that installed outside the production plant is gaining heat from ambient at a rate of 26 kW. As the pipe section is uniform and has no sharp edge, there is no pressure drop as the fluid flows through it. Given respective molar mass of CH4, C2H6, C3H8, and C4H10 is 16.04 g/mol, 30.07 g/mol, 44.1 g/mol, and 58.12 g/mol. Take Ru as 8.314 kJ/kmol-K. We Electrical heater sh Control volume Fan Electrical motor (a) Determine the total pressure of the system, the mole fraction of each gas species, and the molar mass of the gas mixture. (4 Marks) (b) Calculate the change of specific enthalpy of the gas mixture. (6 Marks) Table C.1: Heat Capacities of Gases in the Ideal-Gas State Constants in equation Cp/Ru = A + BT + CT2 + DT for T (K) from 298 K to Tmax Chemical species Tmax pz00/Ru A 10 B 106 C 10-s D Methane Ethane Propane n-Butane CH4 CH6 C3Hg C4H10 1500 1500 4.217 6.369 9.011 11.928 1.702 1.131 1.213 1.935 9.081 19.225 28.785 36.915 -2.164 -5.561 -8.824 -11.402 1500 1500 (c) Calculate the electrical power of electrical heater and the shaft power of the fan. (4 Marks) (d) Determine the mass flow rate of the gas mixture in this pipe section. (6 Marks)
Step by Step Solution
★★★★★
3.38 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


