Answered step by step
Verified Expert Solution
Question
1 Approved Answer
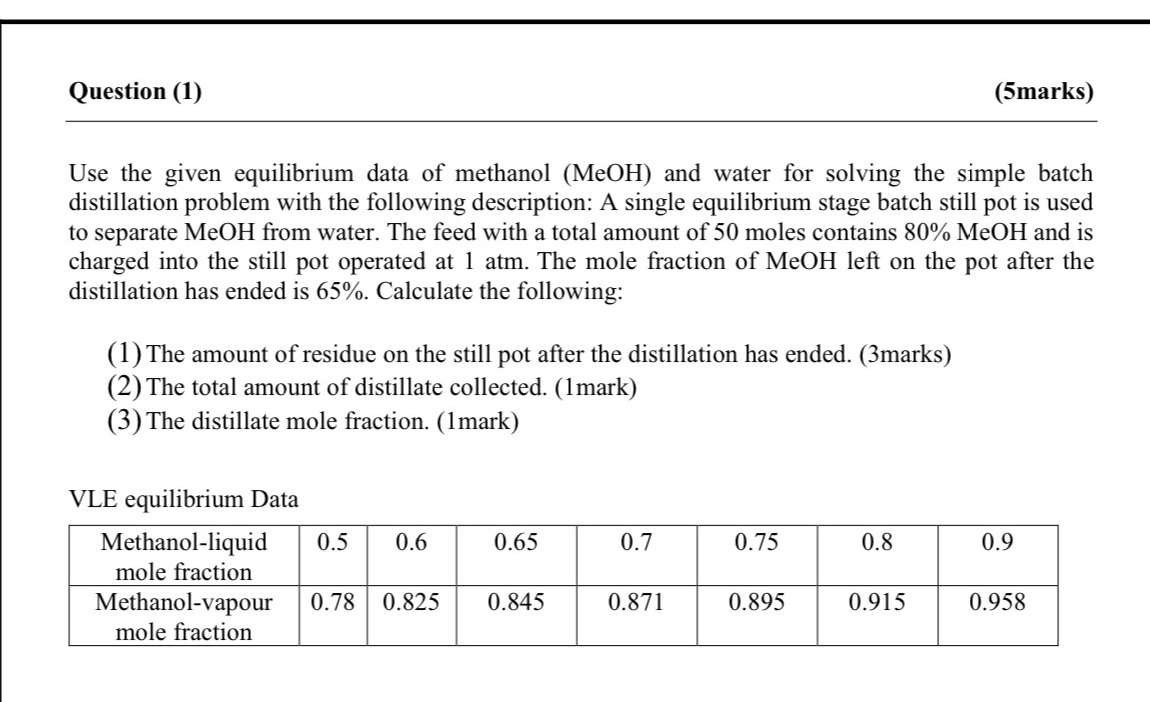
Question ( 1 ) ( 5 marks ) Use the given equilibrium data of methanol ( M e O H ) and water for solving
Question
marks
Use the given equilibrium data of methanol and water for solving the simple batch distillation problem with the following description: A single equilibrium stage batch still pot is used to separate MeOH from water. The feed with a total amount of moles contains MeOH and is charged into the still pot operated at atm. The mole fraction of MeOH left on the pot after the distillation has ended is Calculate the following:
The amount of residue on the still pot after the distillation has ended. marks
The total amount of distillate collected. mark
The distillate mole fraction. mark
VLE equilibrium Data
tabletableMethanolliquidmole fractiontableMethanolvapourmole fraction
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


