Answered step by step
Verified Expert Solution
Question
1 Approved Answer
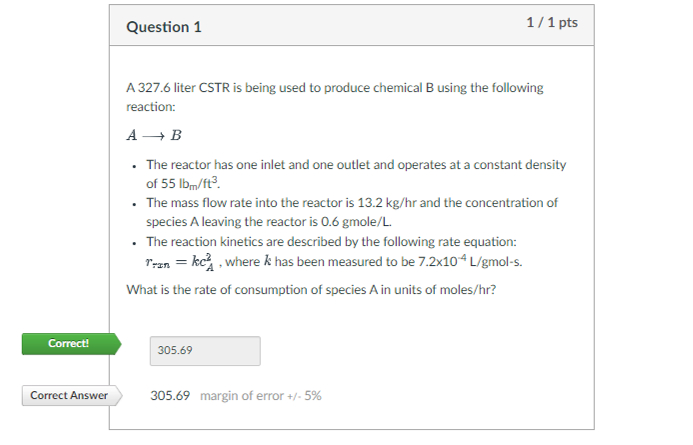
Question 1 A 3 2 7 . 6 liter CSTR is being used to produce chemical B using the following reaction: AlongrightarrowB The reactor has
Question
A liter CSTR is being used to produce chemical B using the following
reaction:
AlongrightarrowB
The reactor has one inlet and one outlet and operates at a constant density
of
The mass flow rate into the reactor is and the concentration of
species A leaving the reactor is gmole
The reaction kinetics are described by the following rate equation:
where has been measured to be mol
What is the rate of consumption of species in units of moles
Correct answer is already given in the question I need complete stepwise solution and reason how he got the shown correct answer Please dont use the chat gpt to get the answer beacuse it gives incorrect answer correct answer will be upvoted and appreciated and incorrect answer will be reported
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


