Answered step by step
Verified Expert Solution
Question
1 Approved Answer
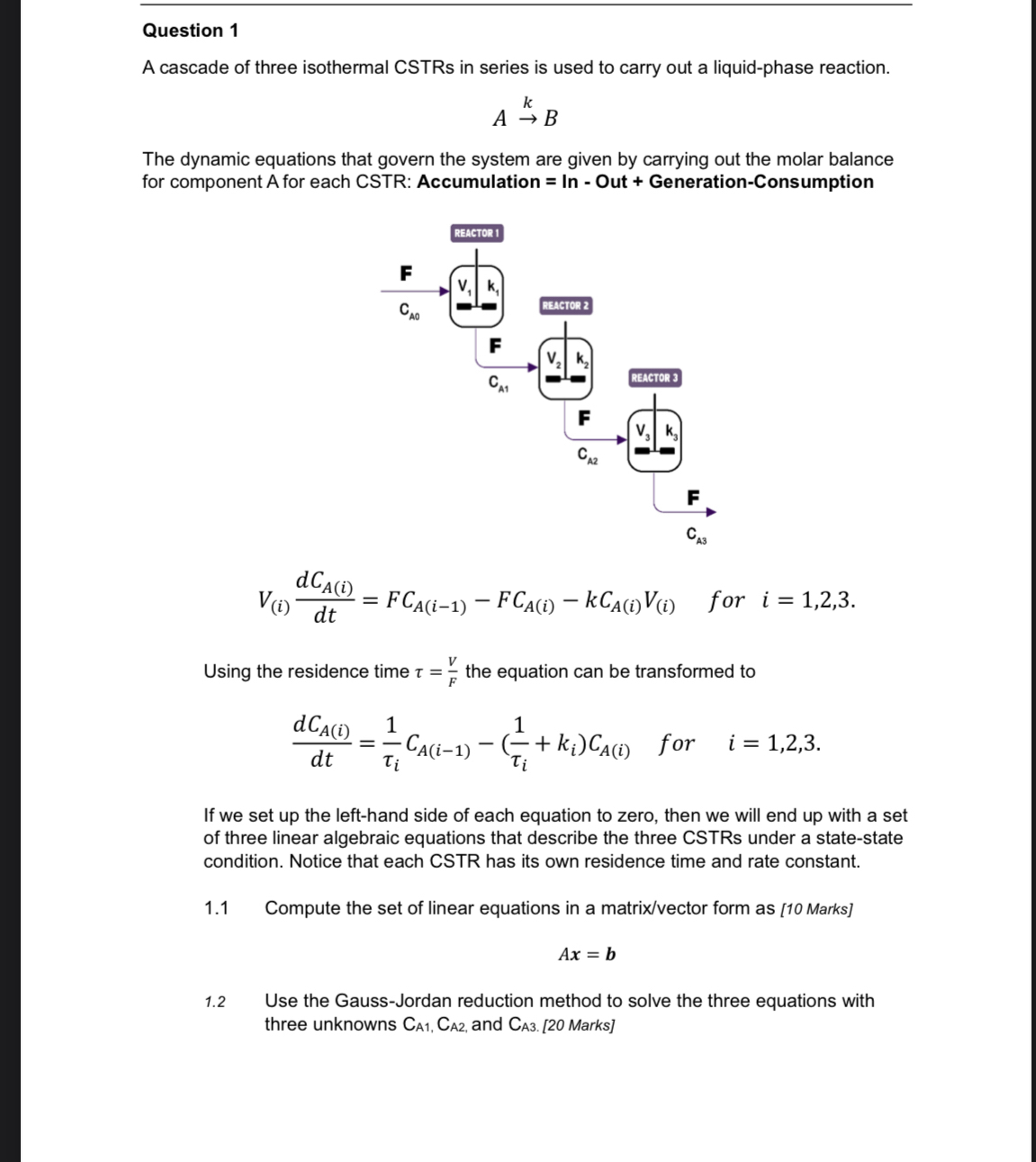
Question 1 A cascade of three isothermal CSTRs in series is used to carry out a liquid - phase reaction. A k B The dynamic
Question
A cascade of three isothermal CSTRs in series is used to carry out a liquidphase reaction.
The dynamic equations that govern the system are given by carrying out the molar balance for component A for each CSTR: Accumulation In Out GenerationConsumption
for
Using the residence time the equation can be transformed to
for
If we set up the lefthand side of each equation to zero, then we will end up with a set of three linear algebraic equations that describe the three CSTRs under a statestate condition. Notice that each CSTR has its own residence time and rate constant.
Compute the set of linear equations in a matrixvector form as Marks
Use the GaussJordan reduction method to solve the three equations with three unknowns and Marks
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


