Question 1 Answer choices: pure substance or mixture
Question 2 Choices:
monotomic element diatomic element mixture containing different compounds mixture containing different elements compound
Question 5 Answer Choices:
Y2P G2 Y W GW YP G2Y2 YP2 GY G
Question 9 Answer Choices:
compound Diatomic element monatomic element
Question 10 Choices:
mixture or pure substance it contains all of the same type of substance or it contains many different compounds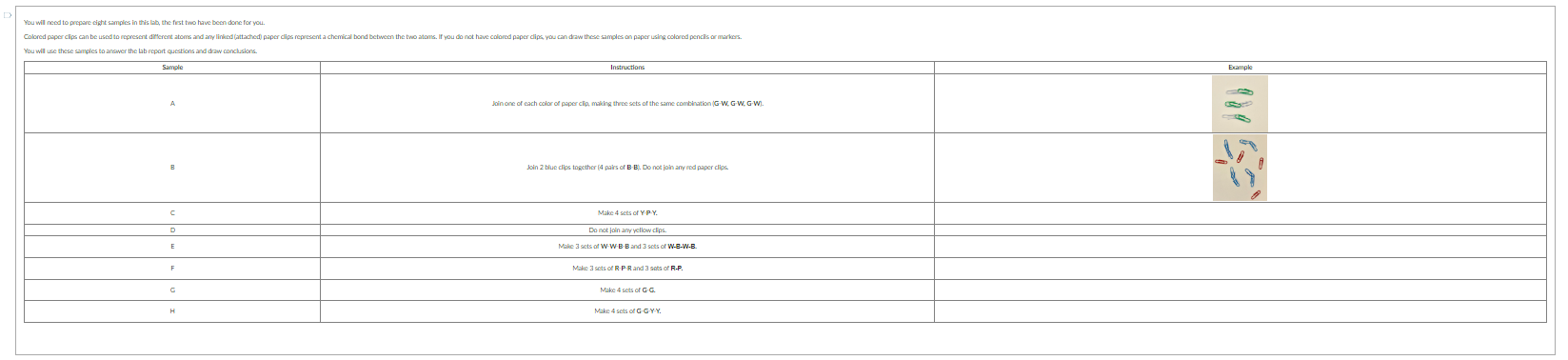
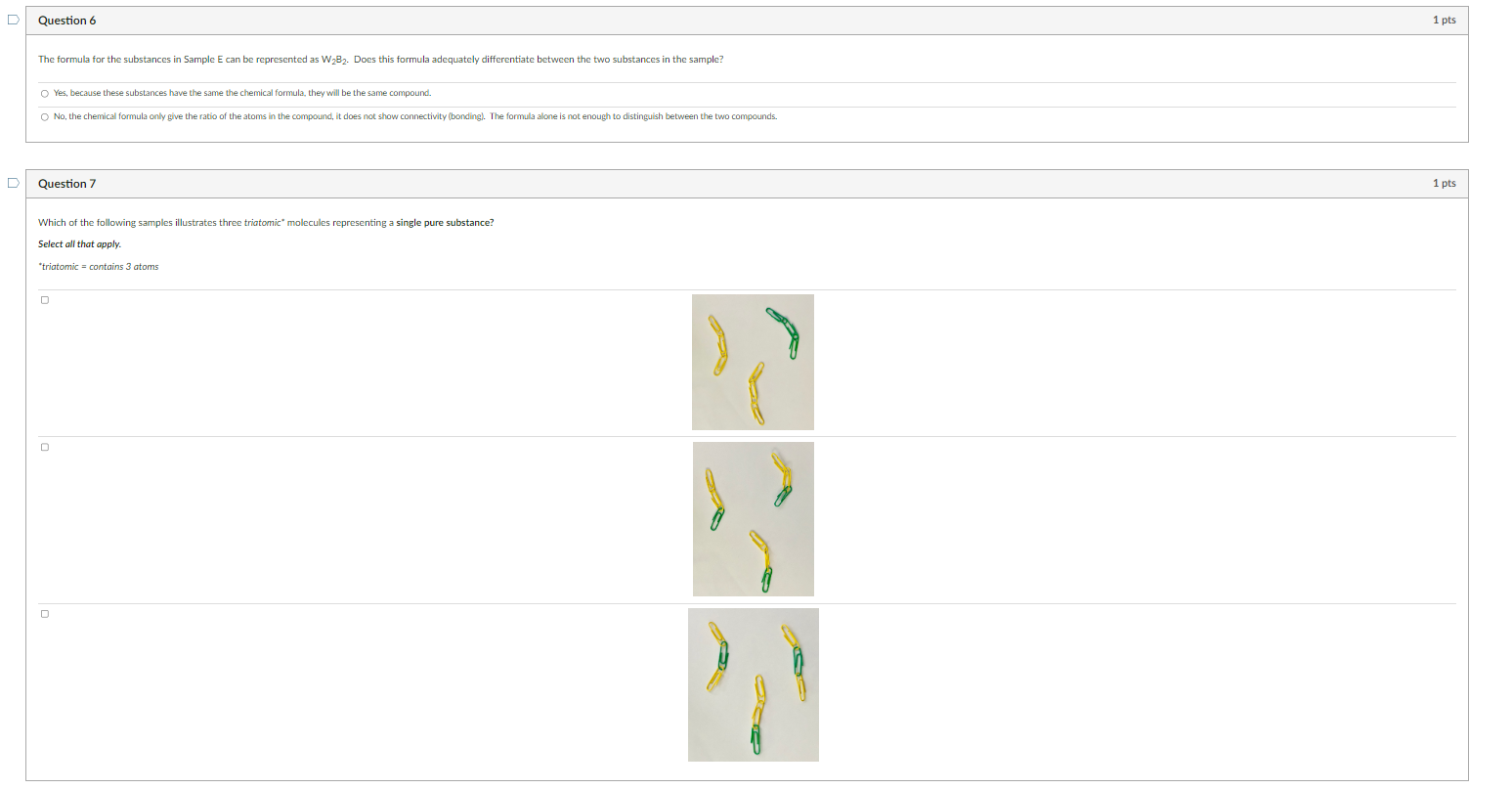
Determine if the samples above represent a pure substance or a mixture. Sample A: Sample B: Sample C: Sample D: Sample E: Sample F: Sample G: Sample H: Question 2 Match the sample to the description that best classifies its makeup. Some descriptions may be used more than once. Sample A Sample B Sample C Sample D Sample E Sample F Which of the following samples does not contain any molecules? Select all that apply. G B A D Question 4 Which sample contains molecules that are isomers? The formula for the substances in Sample E can be represented as W2B2. Does this formula adequately differentiate between the two substances in the sample? Yes, because these substances have the same the chemical formula, they will be the same compound. No, the chemical formula only give the ratio of the atoms in the compound, it does not show connectivity (bonding). The formula alone is not enough to distingulsh between the two compounds. Question 7 Which of the following samples illustrates three triatomic" molecules representing a single pure substance? Select all that apply. "triatomic = contains 3 atoms If Sample A and Sample F were physically combined, what would you expect the result to be? A pure substance containing a new compound. Amikture containing isomers. A mixture containing two different compounds. Amixture containing three different compounds. Question 9 The main components of the air that we breathe are nitrogen (N2), oxygen (O2), Argon (Ar), carbon dioxide (CO2), and water vapor (H2O). Correctly classify each component of air. Nitrogen (N2) : Oxygen (O2) : Argon (Ar): Carbon dioxide (CO2) : Water vapor (H2O) : Question 10 Consider "air" as described above in Question 9. Is air a mixture or a pure substance? Explain. Determine if the samples above represent a pure substance or a mixture. Sample A: Sample B: Sample C: Sample D: Sample E: Sample F: Sample G: Sample H: Question 2 Match the sample to the description that best classifies its makeup. Some descriptions may be used more than once. Sample A Sample B Sample C Sample D Sample E Sample F Which of the following samples does not contain any molecules? Select all that apply. G B A D Question 4 Which sample contains molecules that are isomers? The formula for the substances in Sample E can be represented as W2B2. Does this formula adequately differentiate between the two substances in the sample? Yes, because these substances have the same the chemical formula, they will be the same compound. No, the chemical formula only give the ratio of the atoms in the compound, it does not show connectivity (bonding). The formula alone is not enough to distingulsh between the two compounds. Question 7 Which of the following samples illustrates three triatomic" molecules representing a single pure substance? Select all that apply. "triatomic = contains 3 atoms If Sample A and Sample F were physically combined, what would you expect the result to be? A pure substance containing a new compound. Amikture containing isomers. A mixture containing two different compounds. Amixture containing three different compounds. Question 9 The main components of the air that we breathe are nitrogen (N2), oxygen (O2), Argon (Ar), carbon dioxide (CO2), and water vapor (H2O). Correctly classify each component of air. Nitrogen (N2) : Oxygen (O2) : Argon (Ar): Carbon dioxide (CO2) : Water vapor (H2O) : Question 10 Consider "air" as described above in Question 9. Is air a mixture or a pure substance? Explain











